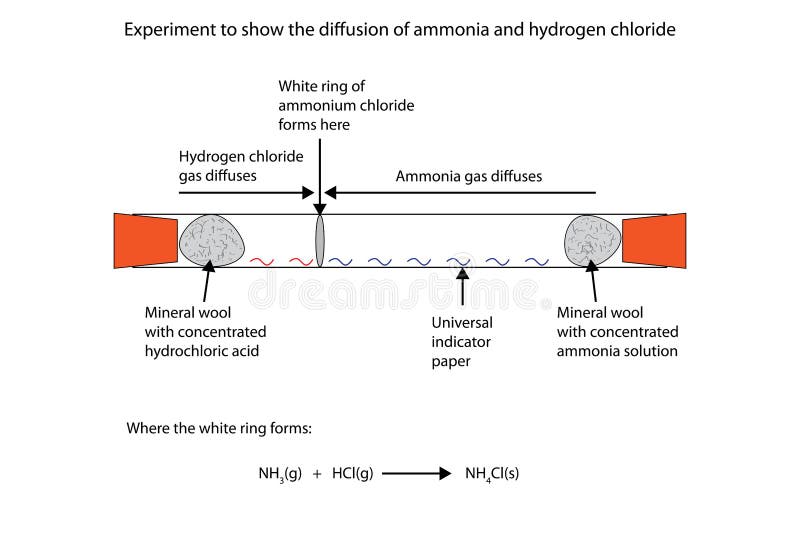
Recommendation Ammonia Gas And Hydrogen Chloride Gas Equation
Ce underset text Ammonia NH_ 3 g underset text Hydrogen chloride HCl_ g - underset text Ammonium chloride NH4Cl_ s.
Ammonia gas and hydrogen chloride gas equation. The chemical equation for the decomposition of ammonia is. 6 Concentrated ammonia solution gives off ammonia gas. Ammonia NH 3 and hydrogen chloride HCl are both colourless gases.
Balance oxygen last since it is present in more than one molecule on the right side of the equation. To happen this second step reaction ammonia is required. Apparatus is set up as shown.
Avail 25 off on study pack. 2 stoppered Erlenmeyer flasks with ammonia and hydrogen chloride gases are opened and the product of the reaction ammonium chloride white smoke will appea. BSolid iron III oxide reacts with hydrogen gas to form solid iron and liquid water.
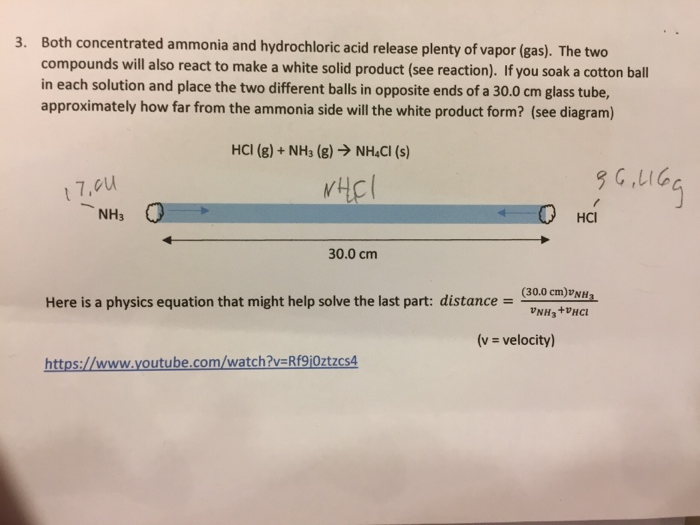
The molar mass of ammonia 17 gm mol -1 and hydrogen chloride 365 gm mol -1. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows. AGaseous hydrogen chloride reacts with oxygen gas to form chlorine gas and liquid water.
Ammonium Chloride is a white solid. It is a combination reaction. 2NH3 -- N2 3H2 a Nitrogen gas is treated with hydrogen gas in the presence of a catalyst at 773 K to form ammonia gas.
Ammonia gas and hydrogen chloride gas react to form the salt ammonium chloride. How many liters of ammonia gas can be formed from 137L of hydrogen gas at 930 degrees Celsius and a pressure of 225atm. CSulfur dioxide gas reacts with oxygen gas to form sulfur trioxide gas.