Neat Balanced Equation For Butane And Oxygen
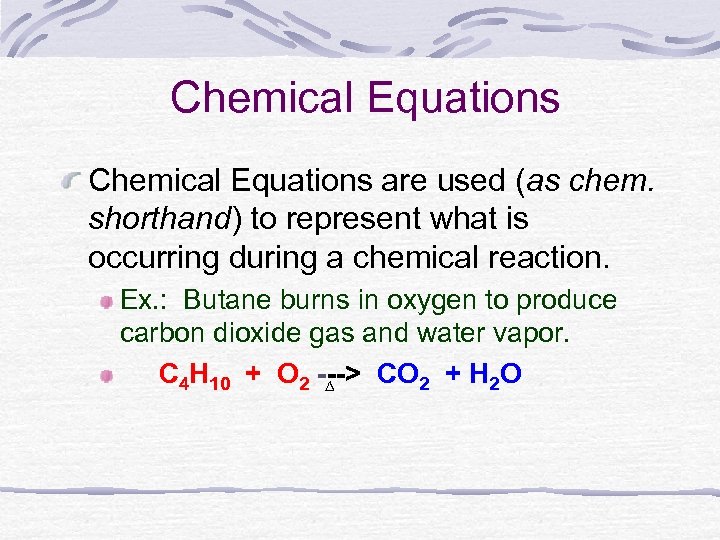
Write the balanced chemical equation for combustion of butane as shown below.
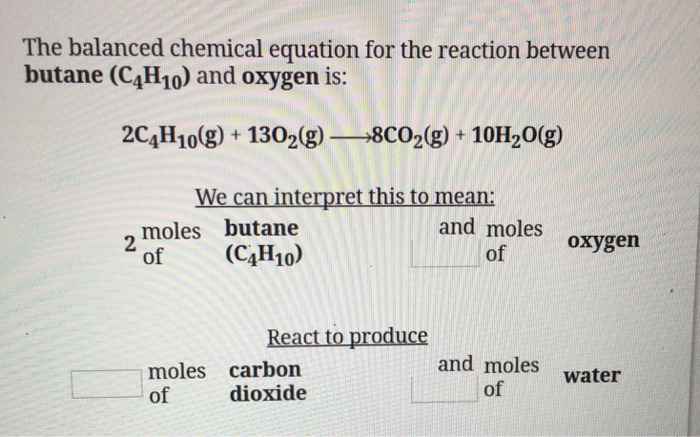
Balanced equation for butane and oxygen. Given the balanced equation for the reaction of butane and oxygen. 0 wt to 0. Given the balanced equation 2 C4H10 13 O2 8 CO2 10 H2O how many moles of water H2O will be produced from 25 moles Of oxygen O2.
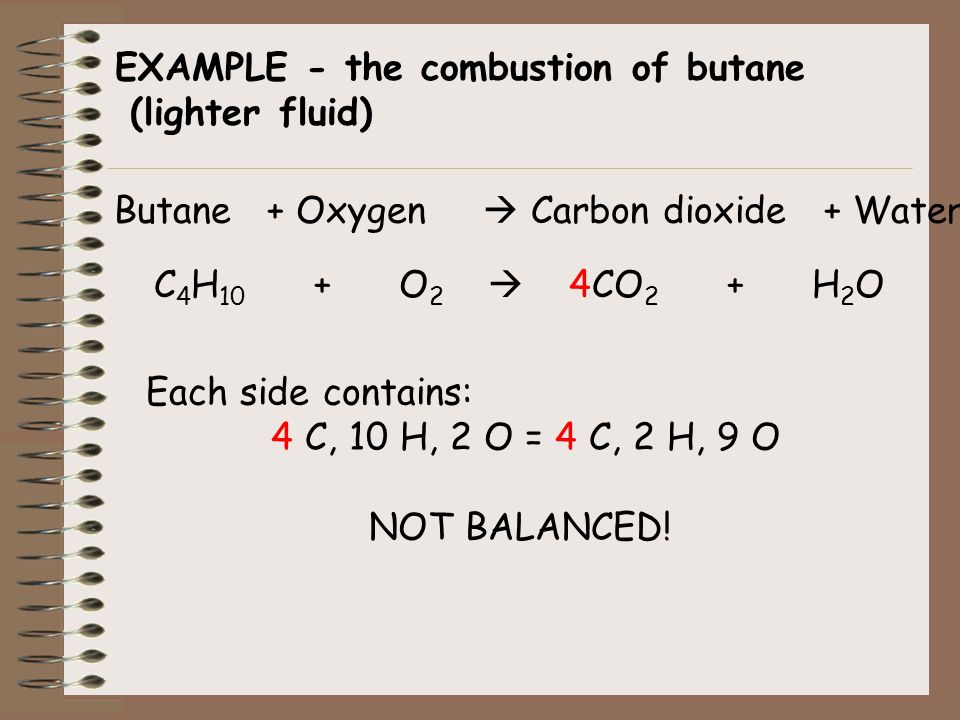
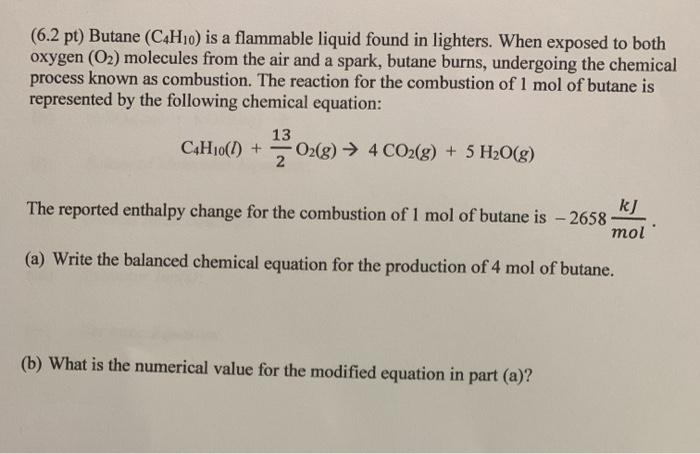
2C4H10g 13O2g 8CO2g 10H2O g. In its unbalanced form the equation for the combustion of butane translates to. Molar mass of butane 58 gmol.
Is butane burning in a butane lighter a chemical reaction. Be sure your answer has the correct number of significant digits in it. Butane is a saturated aliphatic hydrocarbon that contains.
To create a proper balance one may only adjust the numbers of one product or one reactant at a time. In the commercial production of sugar sucrose the product crystals are washed and centrifuged to partial dryness. It will consume first in the balanced equation.
Carbon monoxide can react with oxygen to form carbon dioxide. The ratio of wet sugar to inlet air fed to the dryer is 33 kg wet sugarkg inlet air. The crystals are then sent through a rotary dryer where they are contacted with a hot stream of air that reduces the moisture content from 1.
E Ammonium nitrate Nitrogen Carbon dioxide water. M 32 x 25. The combustion of butane is a reaction between butane and oxygen gas that produces carbon dioxide gas and water.