Recommendation Definition For Balanced Chemical Equation

Law of conservation of mass governs the balancing of a chemical equation.
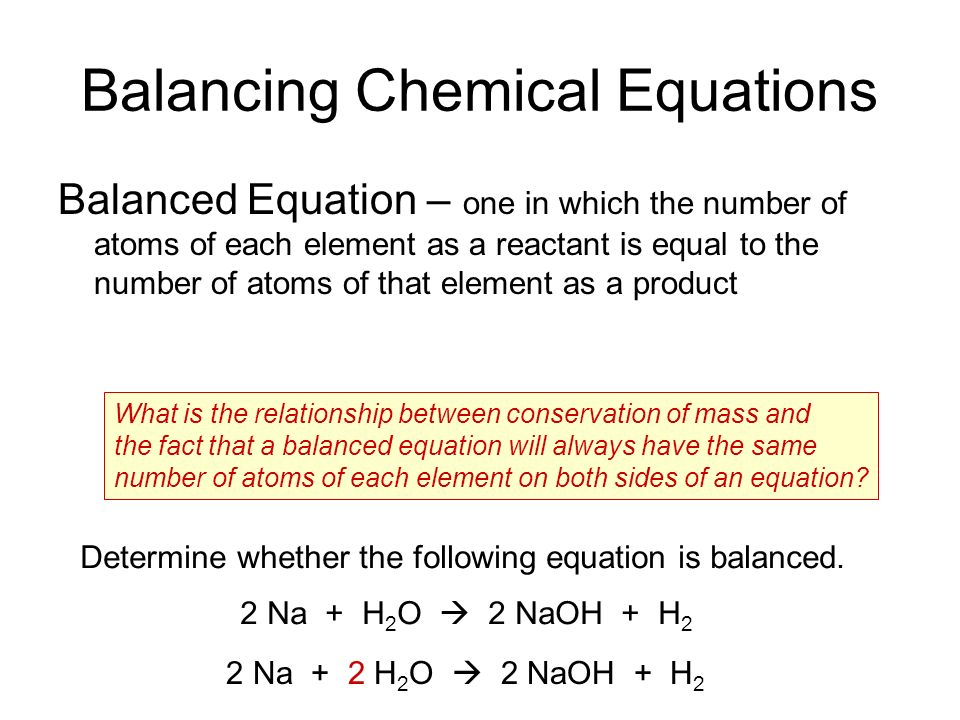
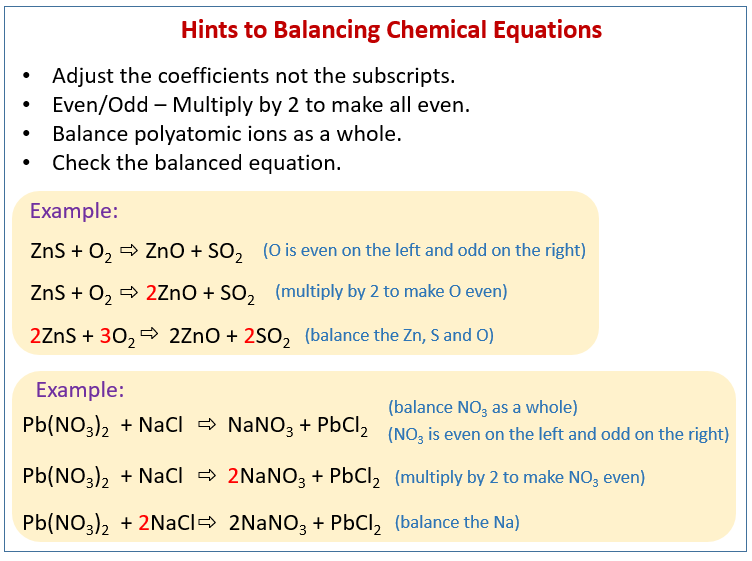
Definition for balanced chemical equation. A balanced chemical equation occurs when the number of the atoms involved in the reactants side is equal to the number of atoms in the products side. The equation contains both qualitative and quantitative information related to the nature and quantity of the substances involved in the chemical reaction. A balanced chemical equation is when the mass of each element of the reactants left side is equal to the mass of each element in the products right side.
Fe Au Co Br C O N F. In other words the mass and the charge are balanced on both sides of the reaction. The two are separated by an arrow symbol usually read as yields and each individual substances chemical formula is separated from others by a plus sign.
Balancing the equation balancing the reaction conservation of charge and mass. What is the definition of balanced chemical equation. ə nst ɪˈkweɪʒ ə n us ˌbæl.
Balanced Chemical equation. Use uppercase for the first character in the element and lowercase for the second character. This is called a balanced equation.
A chemical equation that has an equal amount of each element on the left and right sides 2. It is based on the law of conservation of mass Daltons atomic theory. ə nst ɪˈkweɪʒ ə n a chemical equation that has an equal amount of each element on the left and right sides SMART Vocabulary.
The chemical equation written by balancing total number of atoms of each element in reactants and products It is more informative than the unbalanced chemical equation. Importance of balanced chemical equations - definition It is important to balance chemical equations because there must be an equal number of atoms on both sides of the equation to follow the Law of the Conservation of Mass. A balanced chemical equation shows how many of what molecules react how many of what molecules result and sometimes the state of the substances.