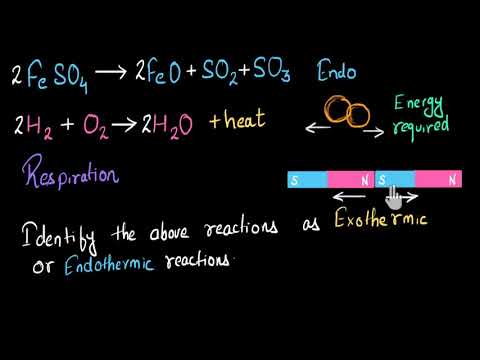Outstanding Endothermic Reaction Examples Class 10

These examples could be written as chemical reactions but are more generally considered to be endothermic or heat-absorbing processes.
Endothermic reaction examples class 10. For any contentservice related issues please contact on this number. And classify the reactions as exothermic or. The chemical equation can be written as follows.
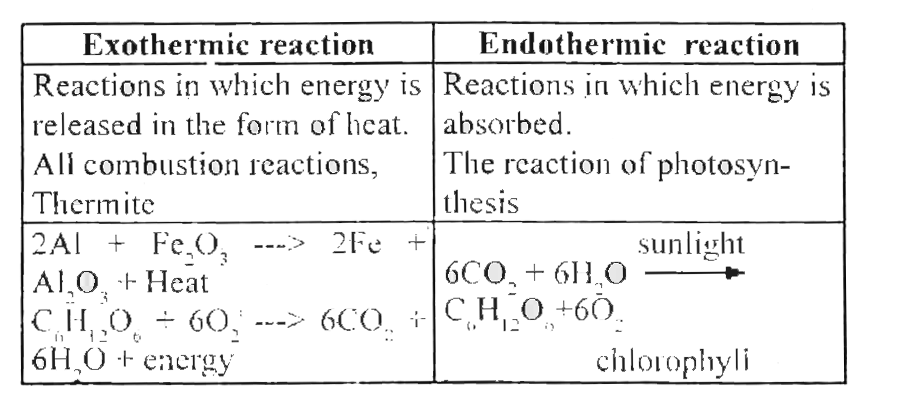
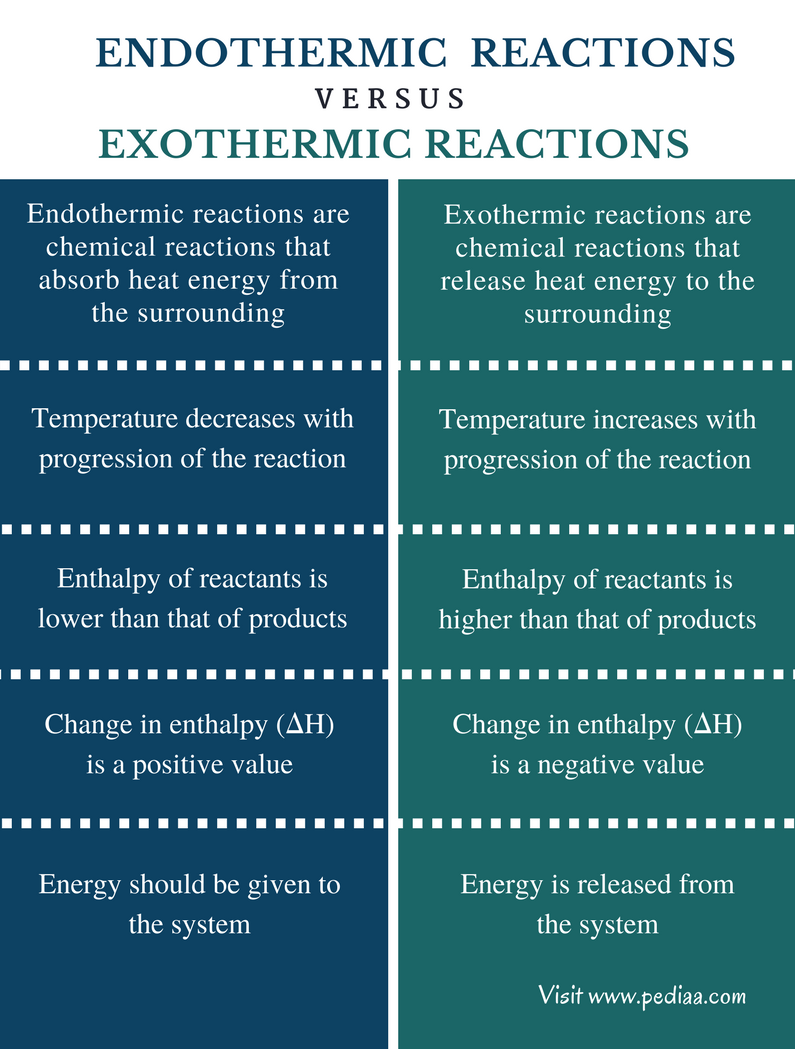
A chemical reaction in which heat is given out is known as exothermic reaction. The chemical reactions which proceed with the absorption of heat energy are called as endothermic reactions. 10 science chemistry double displacement reaction.
The chemical reactions that must absorb energy in order to proceed are known as endothermic reactions. The definition of an endothermic reaction is that it is a chemical reaction that is usually accompanied by the retention of warmth or heat or a living being that produces warmth or heat to keep up its temperature. The salt dissociates into ammonium NH 4 and chloride Cl ions.
When nitrogen and oxygen are heated together to a particular temperature of about 3000 o C nitric oxide gas is formed. The electrolysis of water to form hydrogen and oxygen is also an endothermic reaction. A chemical reaction in which heat energy is absorbed is known as endothermic.
Name two examples of exothermic reactions name two examples of endothermic reactions name two examples of animal species name two examples of heat stabilizers name two examples of plasticizers name two examples of igneous rocks name two examples of lubricants answers to pals certification test revised gre practice test pdf washington state chiropractic jurisprudence exam. An example of an exothermic reaction is the mixture of sodium and chlorine to yield table salt. Following questions consist of two statements Assertion A and Reason R.
Ammonium nitrate NH 4 NO 3 an important component in. C s 2S s Heat CS 2 l. The reaction of citric acid and sodium hydrogencarbonate.