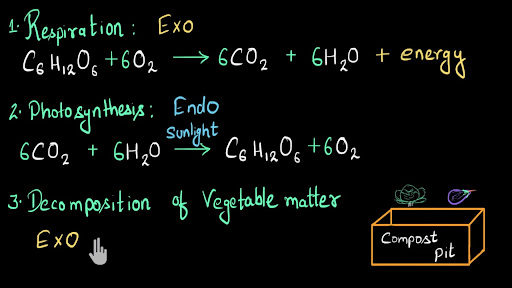Glory Exothermic Examples Equations

All combustion reactions are exothermic reactions.
Exothermic examples equations. Exothermic Reaction An Exothermic reaction is a chemical reaction that involves the release of energy in the form of heat or light. Energy in the form of heat. C6H1206 ---2CH3CH2OH 2CO2 115kjmol.
Created by Ram Prakash. The balanced chemical equations are shown along with the examples 156. Lets categorize them as exothermic or endothermic reactions.
Secondly making ice cubes formation of snow etc are some of the prominent examples. Heat evolved in each of the following. This exothermic reaction can represented as the following equation.
Snow formation in clouds is also an exothermic reaction. Reaction between two gases. These reactions are the opposite of endothermic reactions and can be expressed in a chemical equation as follows.
Endothermic reactions break chemical bonds with. Ultimately the yeast which are facultative fungal organisms provide enzymes that break down sugar molecules while releasing Ethanol and Carbon Dioxide as by-products through the exothermic reaction. Here are some examples of exothermic reactions taking place in laboratories industries and power plants.
B Is oxidation an exothermic or an endothermic reaction. Melting ice evaporation cooking gas molecules photosynthesis are a few examples. When a reaction creates chemical bonds heat energy is released making it exothermic.