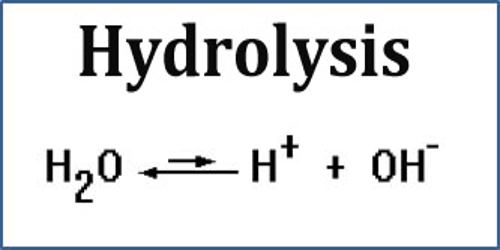Beautiful Work Hydrolysis Chemical Equation

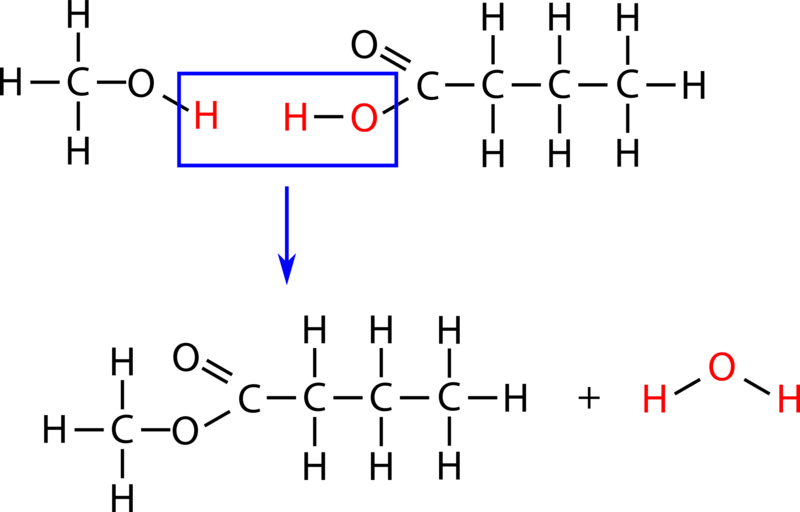
When we talk about the acid hydrolysis of an ester we really mean just adding water to that ester using an acid catalyst and breaking the ester bond COO to.
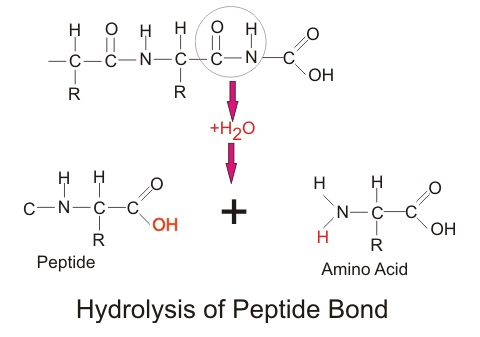
Hydrolysis chemical equation. And typically water is used to break chemical bonds in the other reactant. Hydrolysis reactions result in the breakdown of polymers into monomers by using a water molecule and an enzymatic catalyst. It breaks a chemical bond in the compound.
The main processes of chemical weathering are hydrolysis oxidation and dissolution. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas by a process called electrolysisHydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications. Fe Au Co Br C O N F.
Hydrolysis involving ionic compounds may be illustrated by the chemical changes occurring in an aqueous solution of the salt sodium acetate. Write an equation for the acidic hydrolysis of ethyl butyrate CH 3 CH 2 CH 2 COOCH 2 CH 3 and name the products. Net ionic equations for hydrolysis.
Complete the following table by indicating which process is primarily responsible for each of the described chemical weathering changes. Hydrolysis is an organic chemical reaction that involves adding water to break apart molecules. While it may seem that salt solutions would always be neutral they can frequently be either acidic or basic.
A write the chemical reaction showing the hydrolysis of anilinium ion. Equations A salt is an ionic compound that is formed when an acid and a base neutralize each other. Write a chemical equation for the hydrolysis reaction that explains why an aqueous solution of CH3NH3CI is acidic.
Water molecules combine with the. A common type of hydrolysis occurs when a salt of a weak acid. A way to remember the term.