Outrageous Incomplete Combustion Of Acetylene Gas Chemical Equation
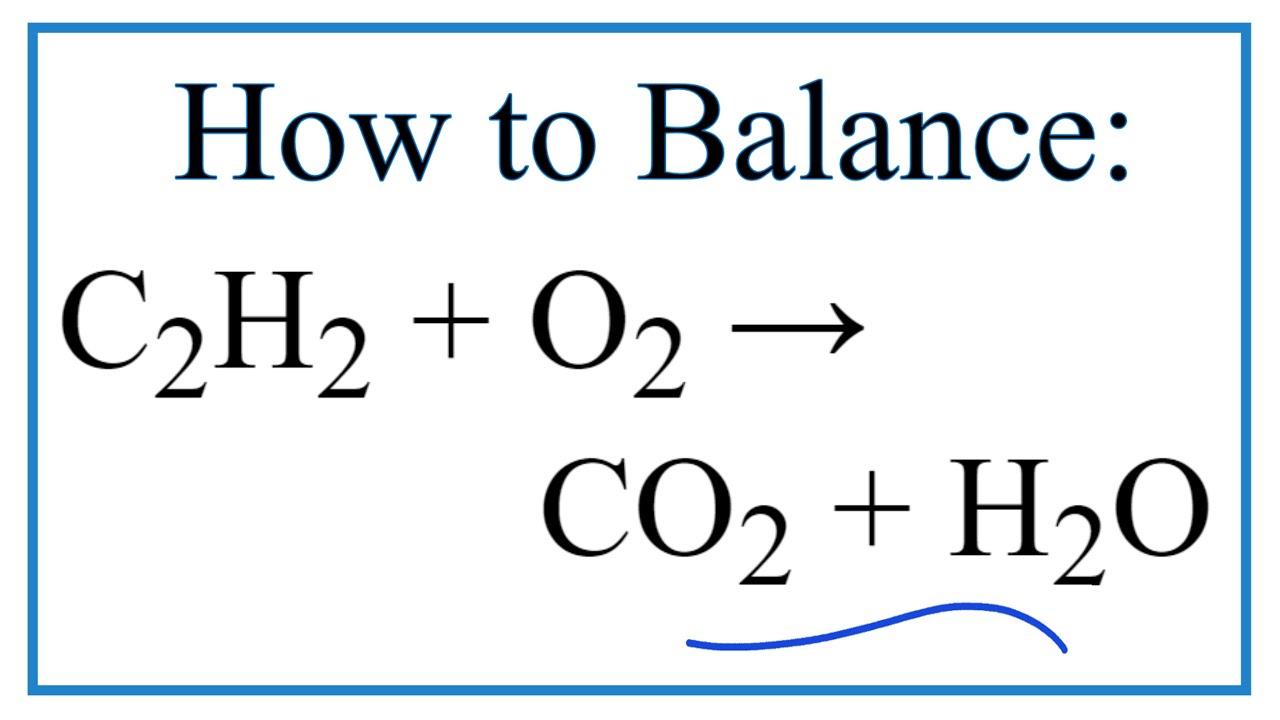
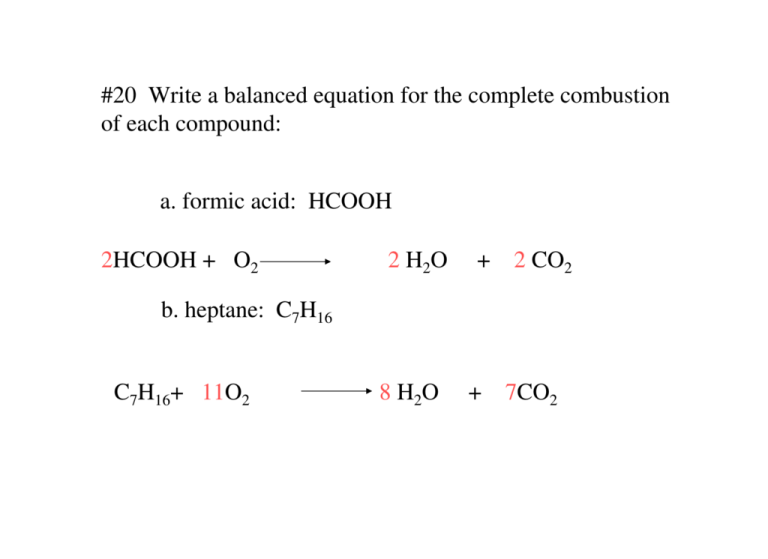
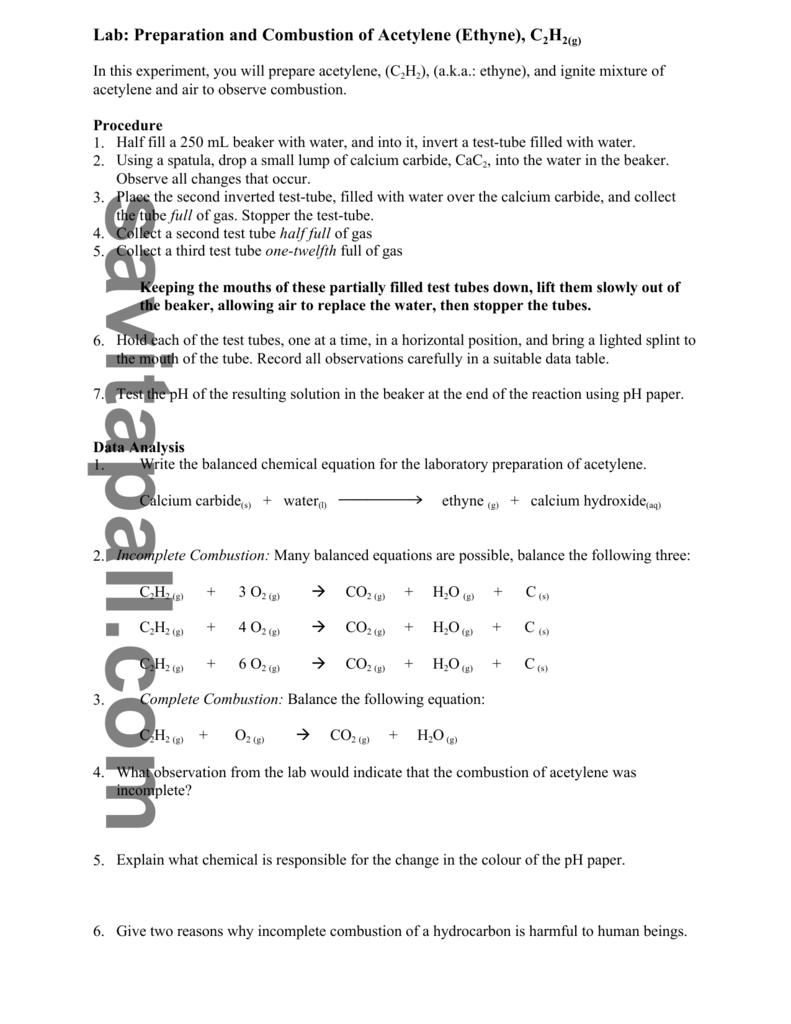
2 C 2 H 2 5 O 2 4 C O 2 2 H 2 Oyoull get complete combustion when acetylene and oxygen are mixed in a 25 mole or volume ratio.
Incomplete combustion of acetylene gas chemical equation. 4 CO2 2 H2O. The equation is specific to the fuel but the general form is. Acetylene is a hydrocarbon consisting of two carbon atoms and two hydrogen atoms.
Balanced Chemical Equation For Incomplete Combustion Of Acetylene Gas. These observations combined with information gathered from the chemical equation indicate that oxygen is the limiting reagent. A Write a balanced chemical equation for the.
The incomplete combustion products will appear as soot and carbon monoxide. 2C_2H_2 g 5O_2 g. In complete combustion of acetylene gas C2H2 C2 H 2 4 302 2 C0 HO 07 1 02 -Y 2 2C HO an a on complete combustion it gives coz while in by on incomplete.
If you have a higher ratio than that combustion will be incomplete. In the presence of a flame acetylene reacts with oxygen to form carbon dioxide and water. The balanced chemical reaction in Equation 2 represents the complete combustion of acetylene to produce carbon dioxide and water.
Complete combustion of a hydrocarbon occurs when oxygen gas in the surrounding air mixes completely and is. Balanced Chemical Equation For The Incomplete Combustion Of Acetylene Gas. Improving primary secondary and tertiary air distribution this includes verification when a boiler is commissioned or after major modifications.
The combustion reaction of acetylene gas is represented by this equation. Complete combustion does NOT give carbon monoxide or soot. Combustion of the acetylene takes place outside the flask due to the lack of available oxygen in the flask.