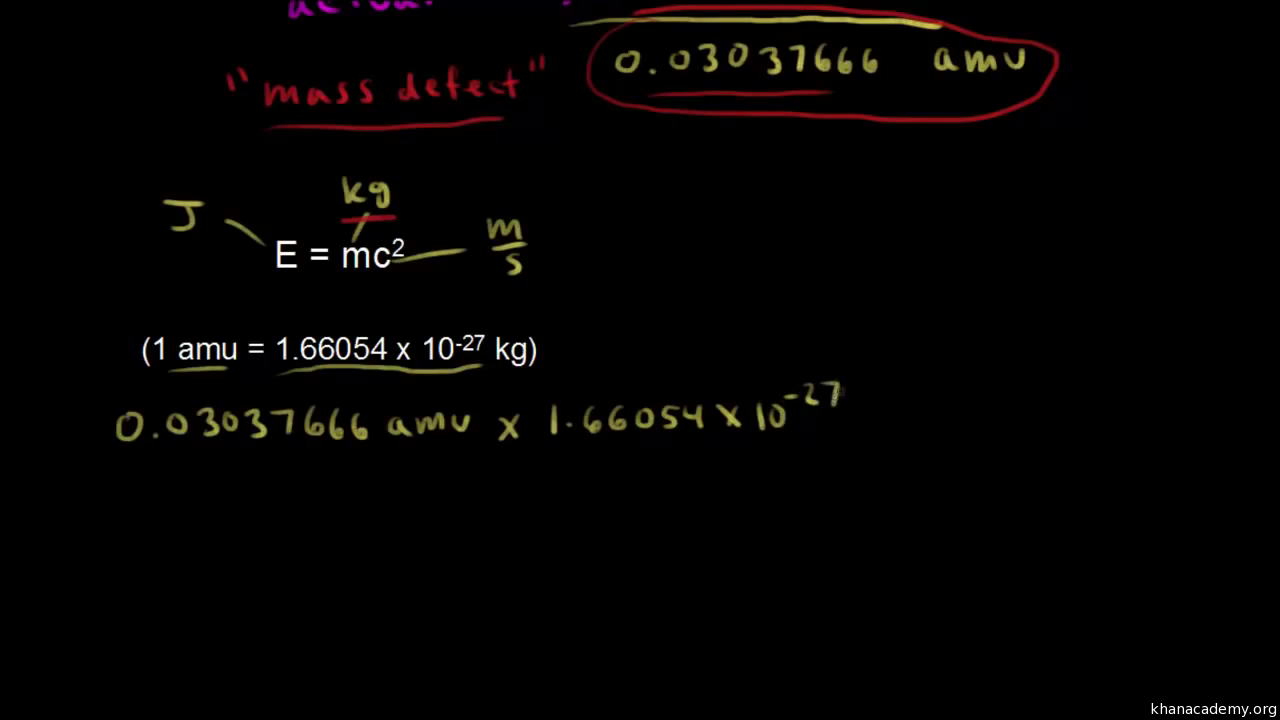Impressive Mass Defect Formula

Standard mass defect is defined as the difference between a compounds or peakss exact mass and its nominal mass.
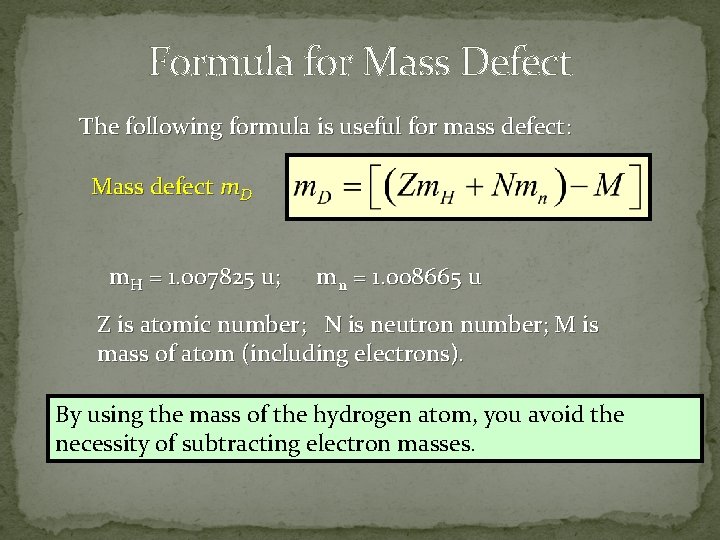
Mass defect formula. Discovered by Albert Einstein in 1905 it can be explained using his formula E mc2 which describes the equivalence of energy and mass. 1 Z m p A Z m n m N Δ m. Recall that energy E and mass m are related by the equation.
Mass Defect Plot serves as a simple tool to visualize peaks in selected document based on their fractional mass or various types of mass defect. This missing mass is known as the mass defect and it accounts for the energy released. E mc 2 The mass of the nucleus is about 1 percent smaller than the mass of its individual protons and neutrons.
According to the formula when energy increases mass and inertia increase. Does an electron have any significant mass. Mass defect which is the mass missing in the resulting nucleus represents the energy released during formation of nucleus The mass defect is given by the formula Here mn mp -represent combined mass of proton and neutron and mo - denotes original mass of atom.
Mass Defect Nuclear binding energy accounts for a noticeable difference between the actual mass of an atoms nucleus and its expected mass based on the sum of the masses of its non-bound components. The actual mass of the nucleus the. Mass defect is the difference between the predicted mass and the actual mass of an atoms nucleus.
Three things need to be known in order to calculate the mass defect. Also let m p be a mass of proton and m n be a mass of neutron present inside it. The binding energy of a system can appear as extra mass which accounts for this difference.
The mass defect arises from the energy released when the nucleons protons and neutrons bind together to form the nucleus. Mass defect also called mass deficit is the difference between the mass of an object and the sum of the masses of its constituent particles. Removing energy reduces mass.