Out Of This World Skeletal Chemical Equation Examples

Furthermore a balanced equation may or may not contain stoichiometric coefficients while a skeleton equation has no stoichiometric coefficients.
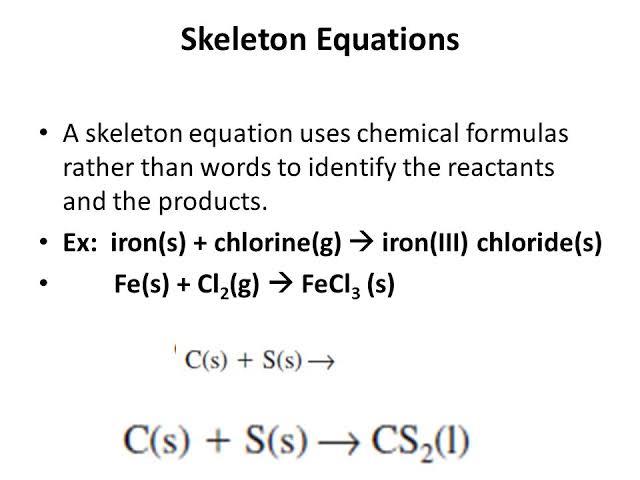
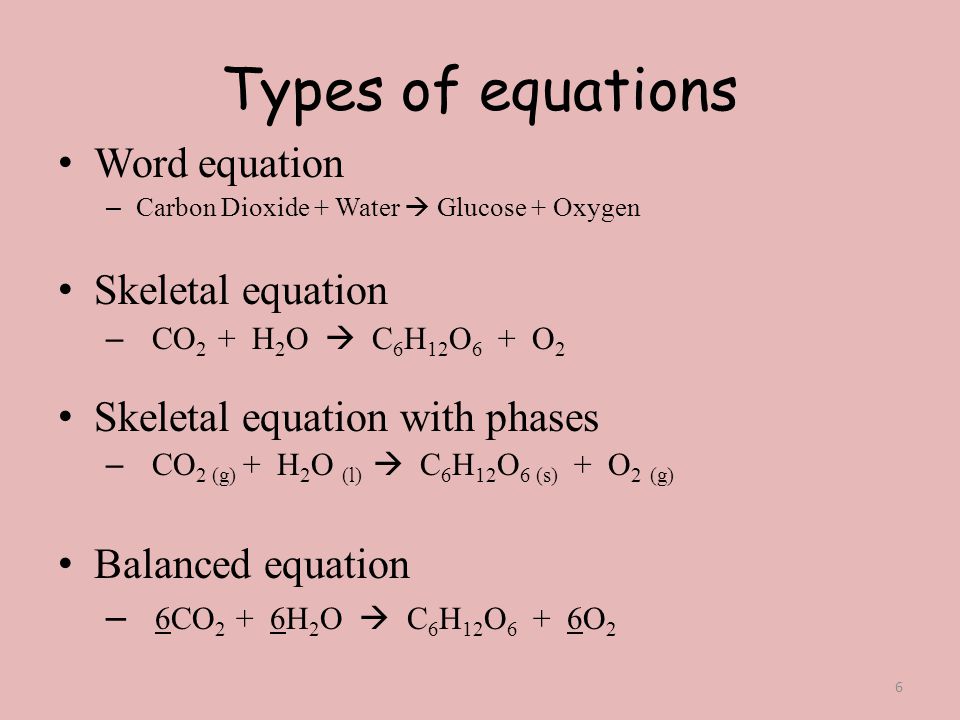
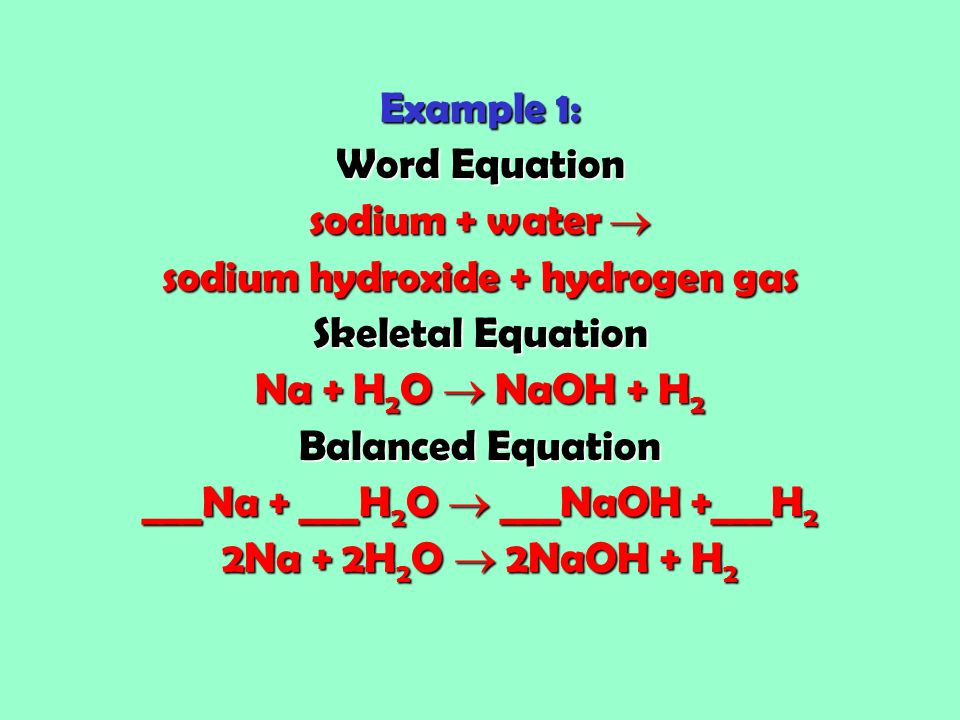
Skeletal chemical equation examples. The chemical equation in which the total number of atoms of each element in reactant and product is not equal is called an unbalanced chemical equation or skeletal chemical equation. Later it has to be balanced by appropriate number of molecules. This equation is also called a skeletal equation.
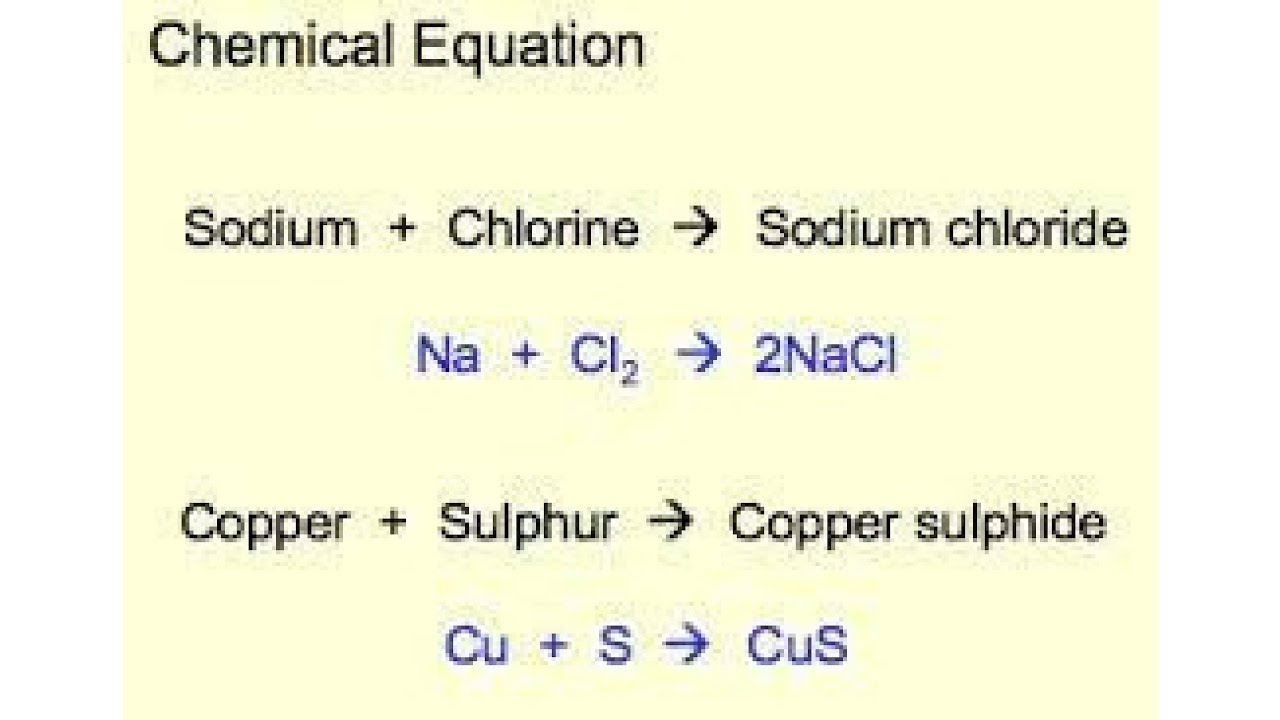
The representation of chemical reaction becomes easy. Writing balanced chemical equations is essential for chemistry classHere are examples of balanced equations you can review or use for homework. Mg O2 MgO its a skeleton equation.
The word equations for a few of these reactions have been provided though most likely youll be asked to provide only the standard chemical equations. Note that if you have 1 of something it does not get a coefficient or subscript. An unbalanced equation is a chemical equation in which the total number of atoms of each element on the reactant side is not equal to the number of atoms of the same element on the product side.
Solved Examples for You Question. The simplest examples of skeletal formulae of organic molecules are those of linear alkanes. Equation is unbalanced because the mass is not the same on both sides.
Mg O2 MgO its a skeleton equation. The skeletal formula also called line-angle formula or shorthand formula of an organic compound is a type of molecular structural formula that serves as a shorthand representation of a molecules bonding and some details of its molecular geometryA skeletal formula shows the skeletal structure or skeleton of a molecule which is composed of the skeletal atoms that make up the molecule. H 2 O 2 H 2 O.
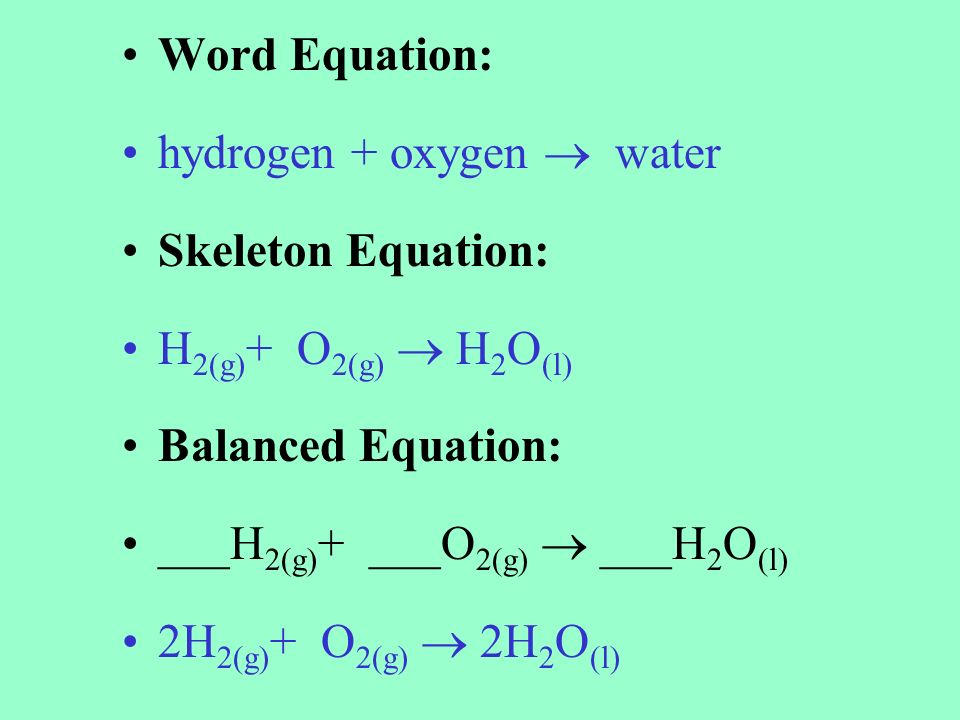
For example we say hydrogen and oxygen react to produce water and we write H2 g O2 g H2O l This is a skeletal equation because it states correctly the molecules H2 and O2 and the compound H2O involved in the equation but it is not balanced. Skeletal equations are equations which show the reactants and the products so formed without balancing them. Start by balancing those elements that occur in only one compound on each side of the equation.