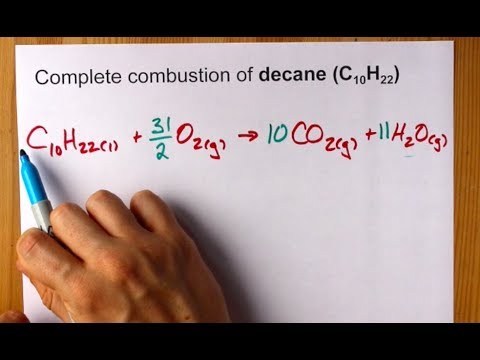
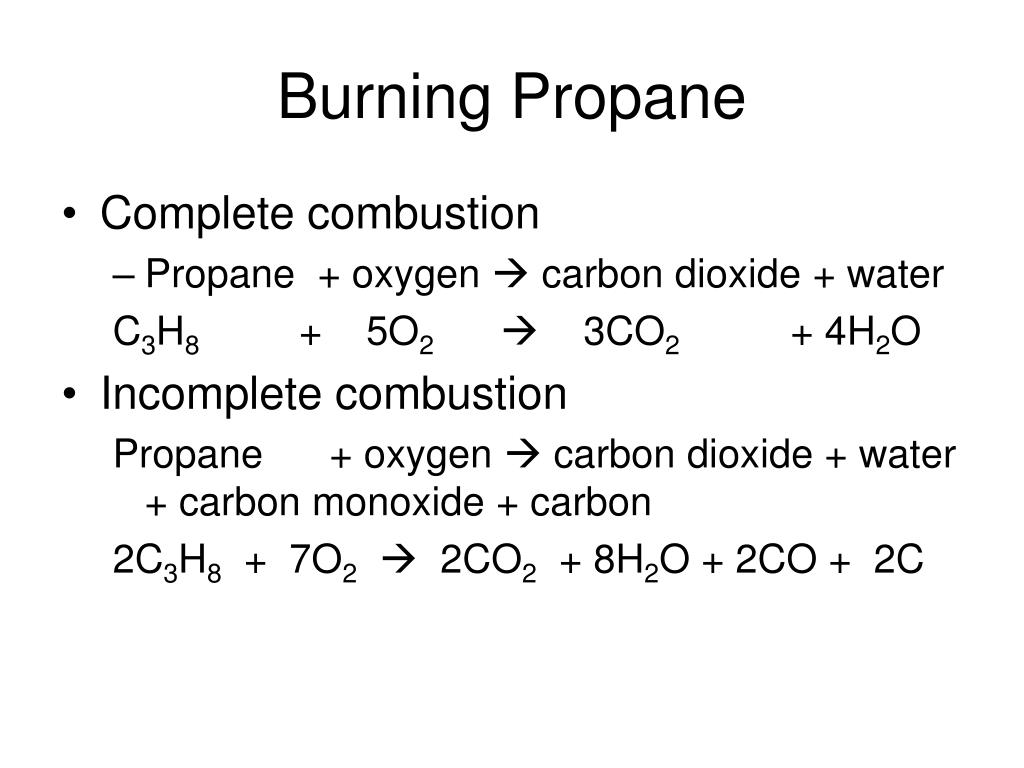
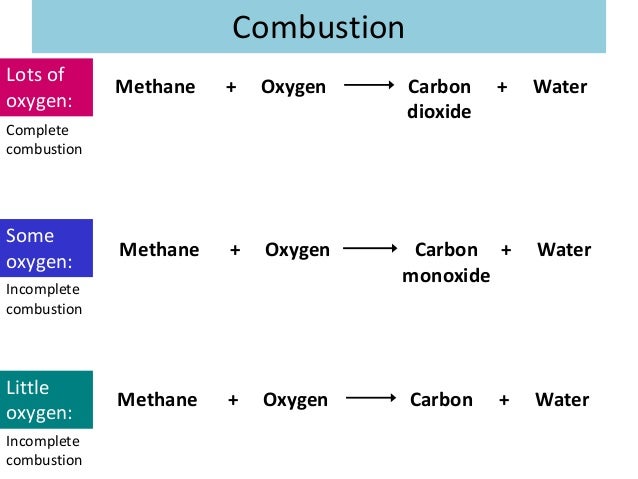
Simple Word Equation For Complete Combustion
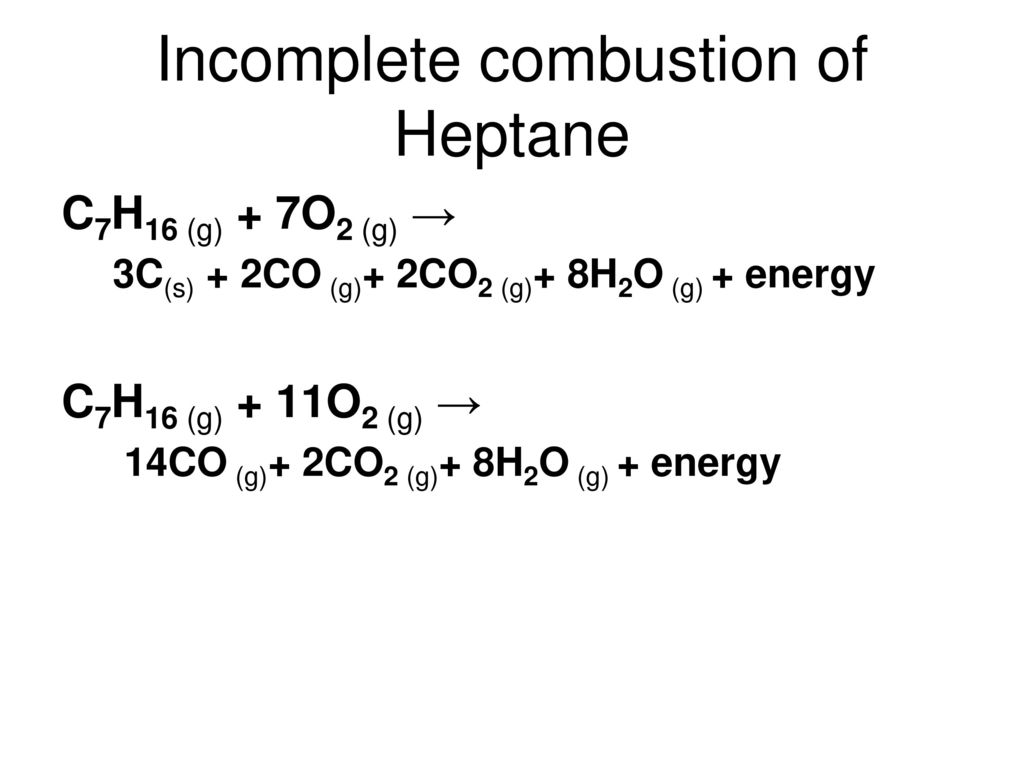
This only apply for the combustion of alkanes.
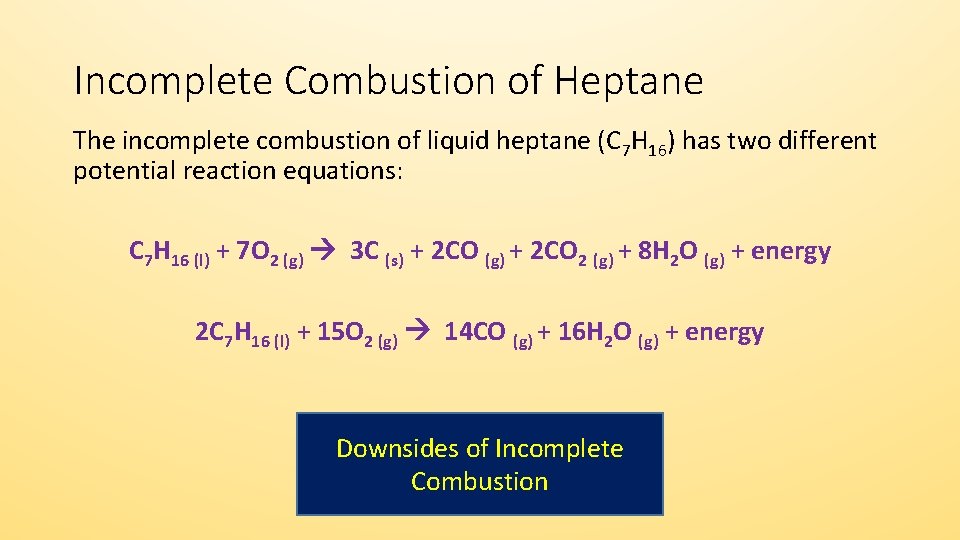
Word equation for complete combustion. Im not sure what you mean by the term word equation However octane is a hydrocarbon molecule consisting of 8 carbon atoms and 18 hydrogen atoms typically written as C8H18. Methane oxygen water carbon dioxide CH4 2O2 2H2O CO2 Candles are made from hydrocarbons. Complete combustion does NOT give carbon monoxide.
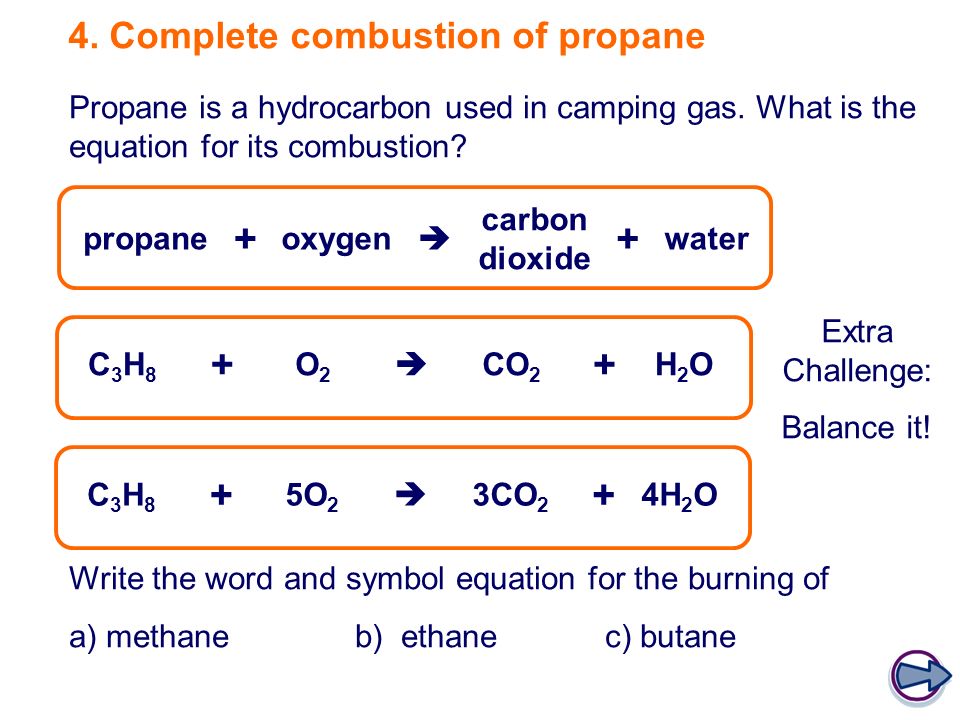
Propane oxygen carbon dioxide water C3H8. The chemical reaction equation for the combustion of octane C 8 H 18 which is one of the primary components of gasoline is 2C 8 H 18 25O 2 16CO 2 18H 2 O. The word equation for wood combustion is that wood in the presence of oxygen and high heat combusts to produce carbon dioxide water vapor heat and ash residue.
The balance equation for the complete combustion. D is free to vary. In the complete combustion of propane equation in the presence of enough oxygen propane burns to form water vapour and carbon dioxide as well as yielding about 25 MJlitre or 49 MJkg of heat.
Wood is made up of cellulose an organic compound that is a polymer or macromolecule. Here are the equations that model its complete combustion. The Complete Combustion of Ethane.
Looking at the equations given you have b c 4 1 d a e b c 2 6 1 d 3 b 2. ΔH combustion Σn ΔH f products - Σn ΔH f reactants 10761 kJmol is the. I have got these bond energies.
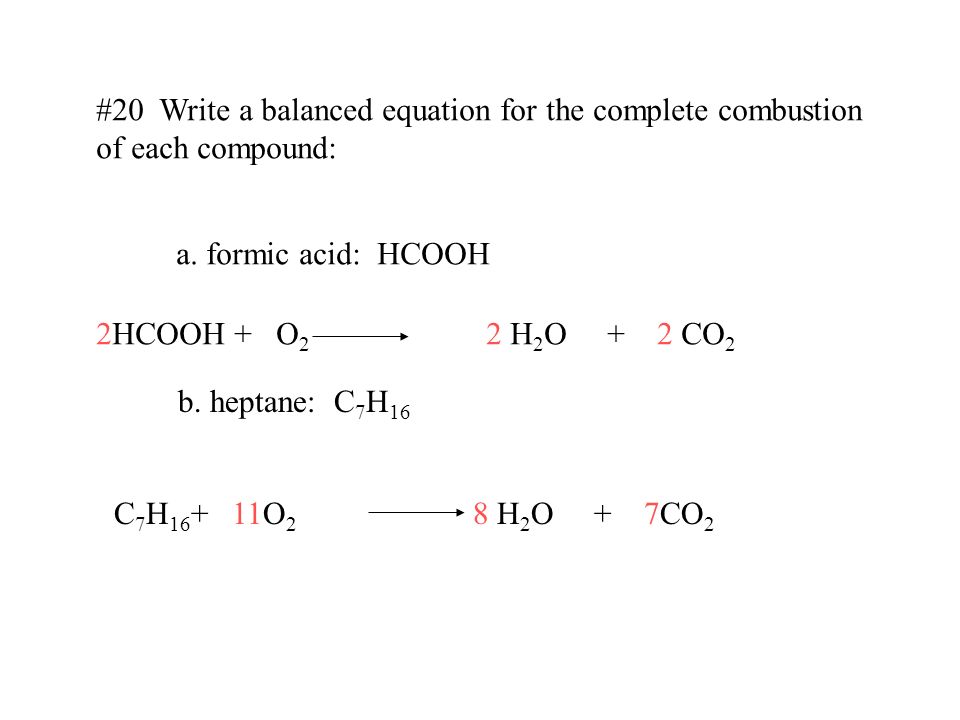
An example of a hydrocarbon is methane CH 4 the main component of natural gas. In general for complete combustion. 2 C 19 H 34 O 2 53 O 2 38 CO 2 34 H 2 O To find the enthalpy of combustion for this reaction the following equation is used.