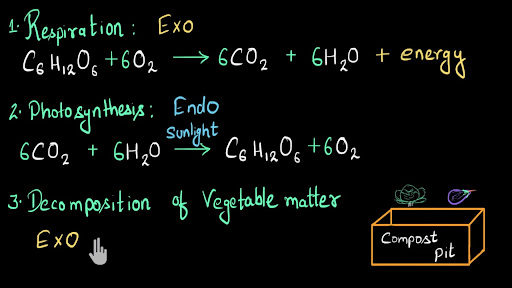Nice Word Equation For Exothermic Reaction

The changes are usually irreversible.
Word equation for exothermic reaction. A chemical reaction is a chemical change in which one or more substances react to form new substances with entirely different properties. On the other hand the potential energy of the product in an endothermic reaction is higher than that of the reactants. As a result the products are likely to be warmer than the reactants.
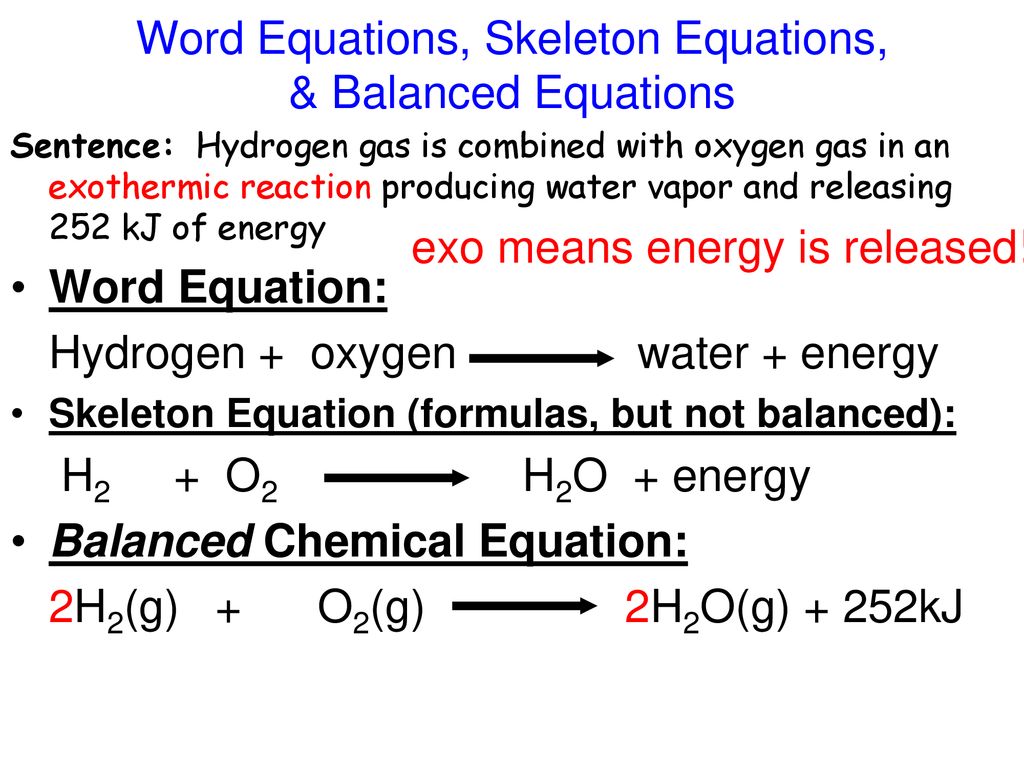
Experimental Procedure for Endothermic Reactions NOTE. Signs of a chemical reaction. The general equation for an exothermic reaction is.
An exergonic reaction is always accompanied by a decrease in free energy of the system. The word equation is. The reaction between magnesium metal and hydrochloric acid is exothermic because it released energy in the form of heat increase of temperature.
Reactants Products Energy. Measurement of temperature change for endothermic reaction part A Experimental Procedure for. Exothermicreactions release energy to the surroundingsso the surroundings get hotter.
Give a Word Equation for the Following Reaction. Exothermic Nuclear Reaction Definition An exothermic reaction is a chemical reaction that releases energy through light or heat. Reactants products energy.
These reactions are the opposite of endothermic reactions and can be expressed in a chemical equation as follows. The energy required to break the bonds of the reactants were more than the energy required to make the new bonds of the products. Using the information above explain why.