Ideal Ammonia + Hcl Equation
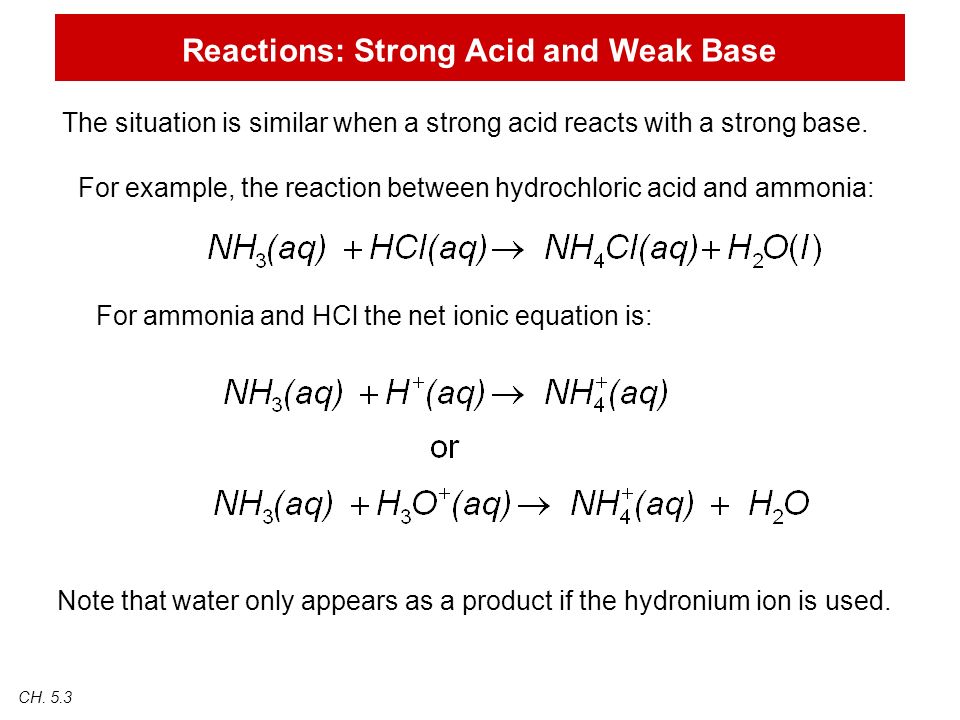
The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows.
Ammonia + hcl equation. Ammonia is a weak base that reacts with hydrochloric acid forming a compound called ammonium chloride. In this video well balance the equation HCl NH3 NH4Cl and provide the correct coefficients for each compoundTo balance HCl NH3 NH4Cl youll need to. If an acid is added to the buffer it is neutralized by the base.
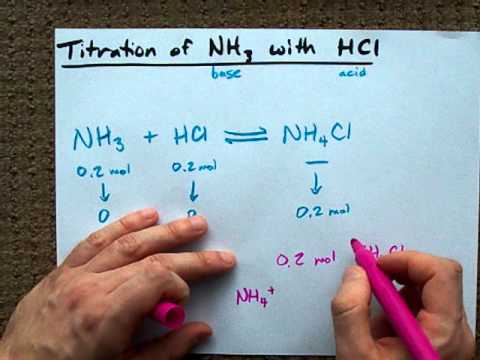
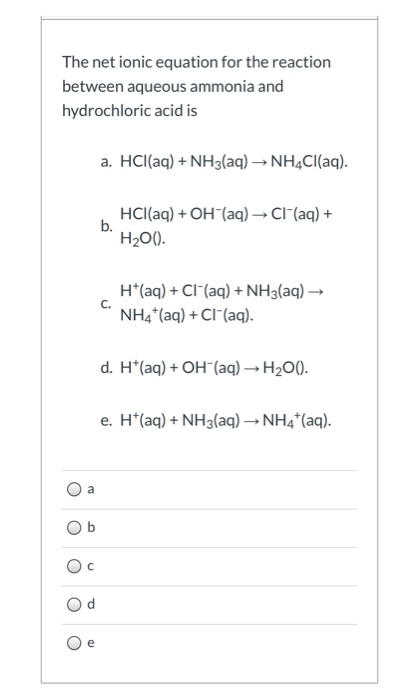
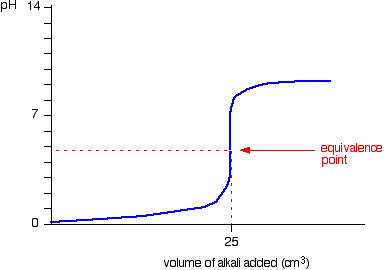
Chemistry The reaction of hydrochloric acid HCl with ammonia NH3 is described by the equation. NH 3 HCl NH 4 Cl. The chemistry of the titration of the weak base ammonia with the strong acid hydrogen chloride is captured by the neutralization reaction NH 3 aq HCl aq NH 4 Cl aq H 2 O l As the essence of the reaction is base acid salt water.
One may also ask what is the chemical reaction for ammonia. A strong acid is an acid which is completely ionized in an aqueous solution. The other product is water.
Similarly if a base for example sodium hydroxide NaOH is added it will react with the acid in the buffer NH 4. Click to see full answer. Of course we could isolate ammonium chloride N H 4Cl if we chose to do so.
Equations with NH4Cl as product. Additionally is HCl a strong acid. The given reaction is the neutralization of ammonium hydroxide with hydrochloric acid.
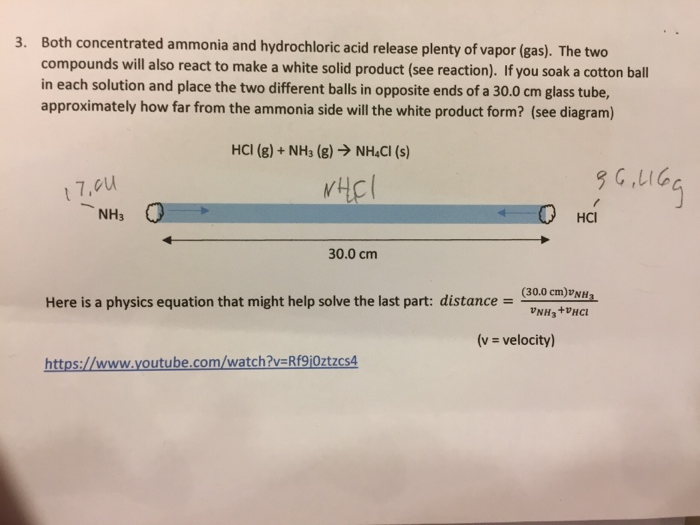
How much hydrochloric acid must be added to react completely. To write the products we combine the anion of the acid with the cation of the base and write the correct formula following the principle of electroneutrality. First we balance the molecular equation.