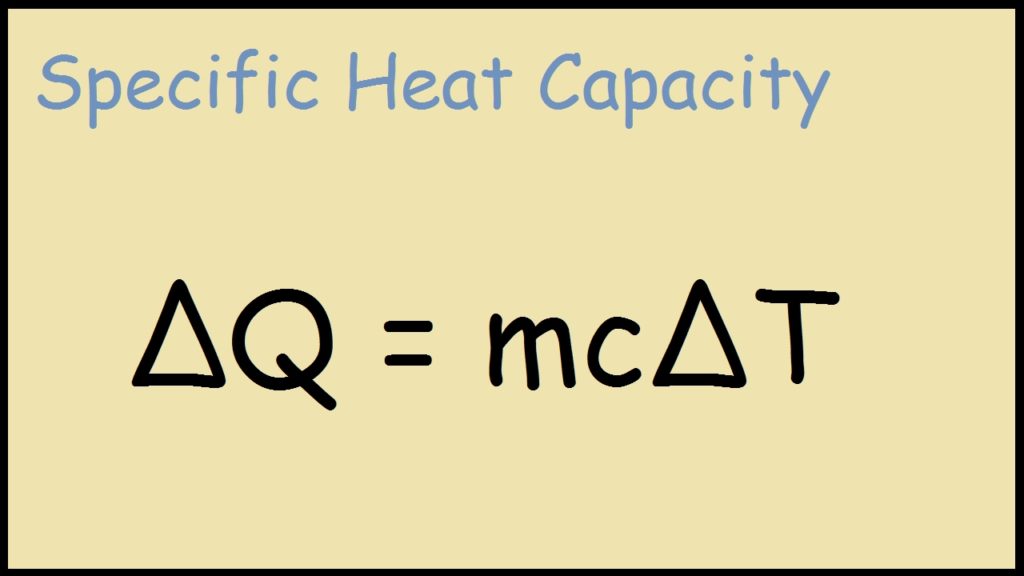Awesome How To Show Heat In Chemical Equation

A catalyst is needed c.
How to show heat in chemical equation. If not steady-state ie transient then 𝐸. Learn the equation for specific heat.
The Heat Equation The heat equation also known as di usion equation describes in typical physical applications the evolution in time of the density uof some quantity such as heat chemical concentration population etc. H qp - PV PV Thus the heat given off or absorbed during a chemical reaction at constant pressure is equal to the change in the enthalpy of the system. Animated plot of the evolution of the temperature in a square metal plate as predicted by the heat equation.
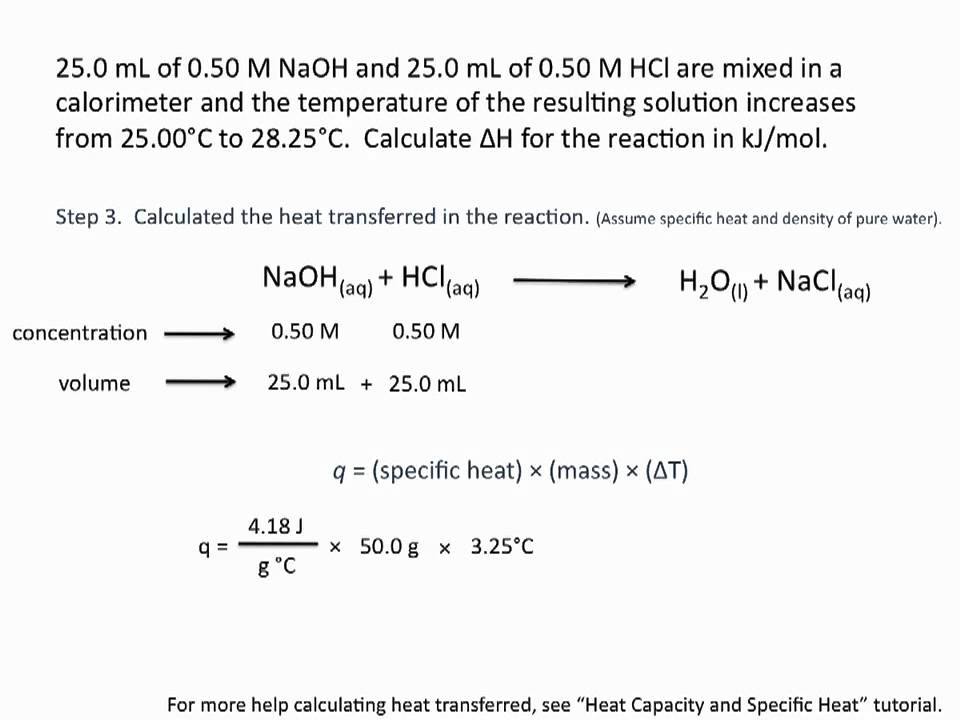
Write the general equation for calculating the standard enthalpy heat of reaction. ΔH oreaction ΣH of products - ΣH of reactants Substitute the values for the standard enthalpy heat of formation of each product and reactant into the equation. In this video I also go over keywords like heat absorbed.
Give directions for naming chemical compounds d. The height and redness indicate the temperature at each point. Practice with some examples.
You first equations is fine although you should probably write calcium carbonate as 2CaCO3 then realise that you have a 2 infront of everything and then ditch it. It is kind of like learning a new language. Heat is supplied to the reaction b.
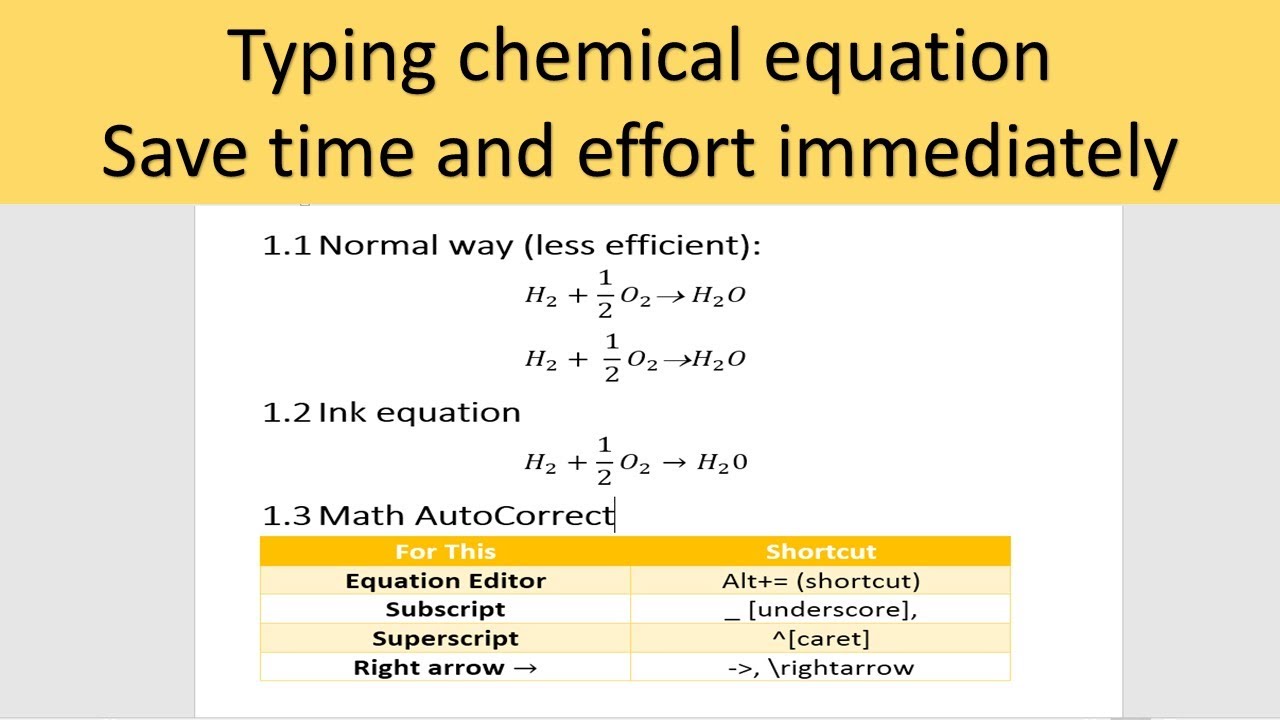
Heat of a chemical reaction can therefore be defined as the heat evolved in the surroundings or absorbed when the reaction takes place at constant pressure and temperature. The total amount of heat absorbed or evolved is measured in Joule J. In order to write the chemical equation it is need to know the formulae of the elements or compounds which are involved in the chemical reaction.