Supreme Root Mean Square Velocity Derivation Pdf

Virial Theorem Simplest 2T U 0 By re-arranging the above equation and making some simple assumptions about TMv22 and UGM2R for galaxies one gets Mv2RG M is the total mass of the galaxy v is the mean velocity of object in the galaxycluster G is Newtons gravitational constant and R is the effective radius size of the object.
Root mean square velocity derivation pdf. Root mean square is also defined as a varying function based on an integral of the squares of. An associated quantity is the root-mean-square r. Similar definitions apply to the lateral and vertical velocities vt and wt.
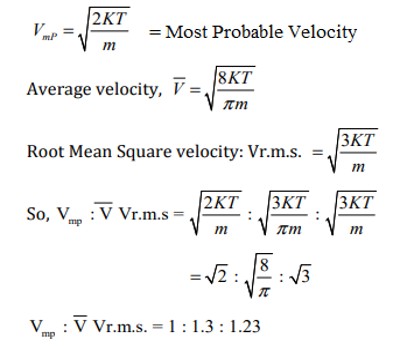
Derivation of Equation of Motion. Gas molecules have different speeds but an average rms or root mean square speed v rms v 2 can be calculated using what is called the equipartition theorem which is 1 2 mv2 3 2 k B T Here T is the absolute temperature of the gas m is the mass of a gas molecule and k B is the Boltzmann constant mentioned before. Figure 77 shows the Maxwell velocity distribution as a function of molecular speed in units of the most probable speed.
Solved Example For Root Mean Square Speed. This is a simple and exact result which says that the mean square displacement of a gas in which there are no collisions grows quadratically in time. T m displaystyle Tm the ratio of.
B a y dt b. Inserting 24 into 22 we get t 3 22 25 vto with is known as the thermal speed. The Mean Value and the Root-Mean-Square Value 142 Introduction Currents and voltages often vary with time and engineers may wish to know the mean value of such a current or voltage over some particular time interval.
Mean square displacement function for the ideal gas. The mean value of a time-varying function is defined in terms of an integral. Because sound waves ultimately propagate via molecular motion it makes sense that they travel at slightly less than the most probable and mean molecular speeds.
For More Chemistry Formulas just check out main pahe of Chemsitry Formulas. Evaluated over the limits of the probability density function ie. We are now in a position to find just how large that increase is for a gaseous substance.