Recommendation 10 Examples Of Endothermic Reactions With Equations

Ammonium nitrate is dissolved in water.
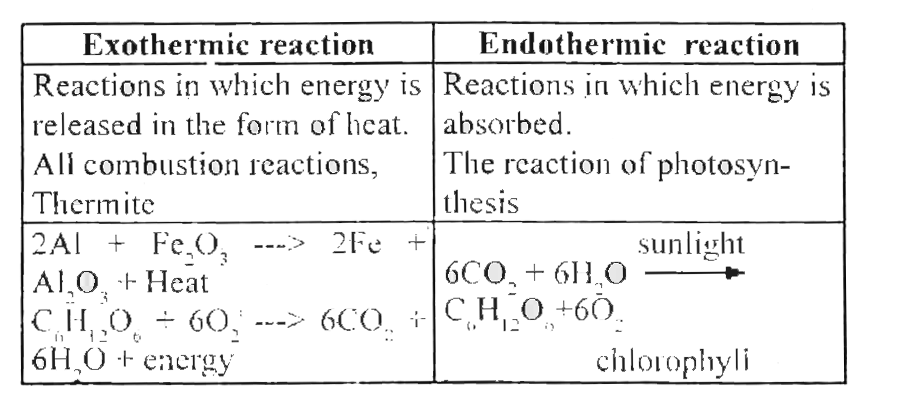
10 examples of endothermic reactions with equations. A few examples of the endothermic process are photosynthesis evaporating liquids melting ice dry ice alkanes cracking thermal decomposition ammonium chloride in water and much more. The endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat. Which of the following is an example of an endothermic reaction.
The chemical equation can be written as follows. This is actually one of the key characteristics of an endothermic reaction. In an endothermic reaction heat is used for the reaction to occur.
Converting frost to water vapor melting boiling and evaporation in general are endothermic processes. Photosynthesis is the chemical reaction that takes place in green plants which uses energy from the sun to change carbon dioxide and water into food that the plant needs to survive and which other organisms such as humans and other animals can eat so that they too can survive. If 10 foresters can cut down 70 trees.
Endothermic Reaction Examples When ammonium chloride NH 4 Cl is dissolved in water an endothermic reaction takes place. An endothermic reaction requires energy while an exothermic reaction releases energy. Which of the following is an example of an endothermic reaction.
Generally but not alway they can be recognized by a drop in temperature in Content. The chemical equation can be written as follows. In chemistry laboratories it is common to deal with endothermic reactions such as hydrolysis of water or the production of molecular chlorine from hydrochloric acid.
NH4Cl s H2O l NH4Cl aq - Heat 10 examples of endothermic reactions with. Characteristics equations and examples A endothermic reaction It i one that to take place mut aborb energy in the form of heat or radiation from it urrounding. Fusion of snow on a warm windshield especially for heavy snow-wet.