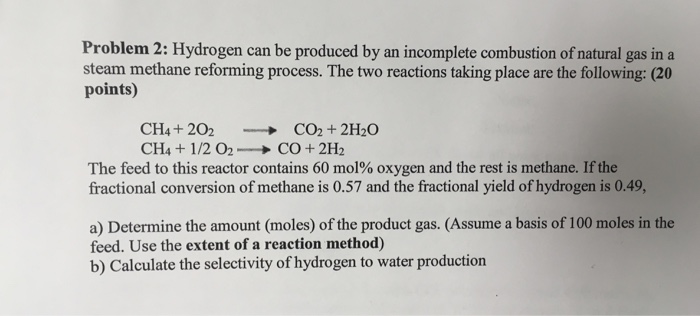
Peerless Incomplete Combustion Of Ch4
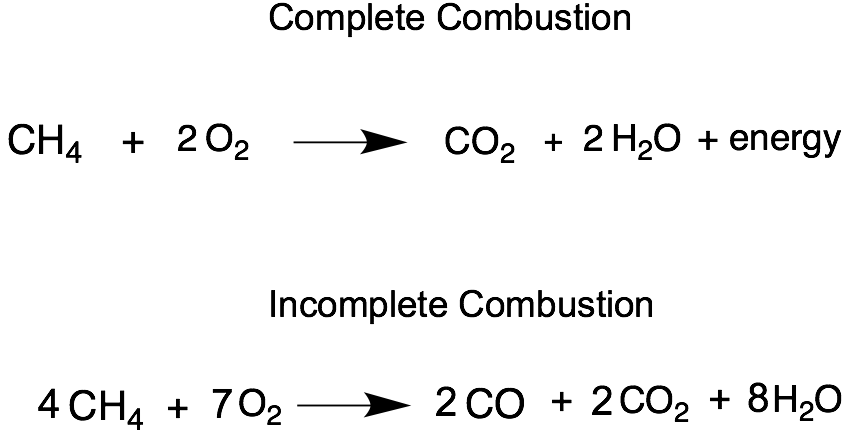
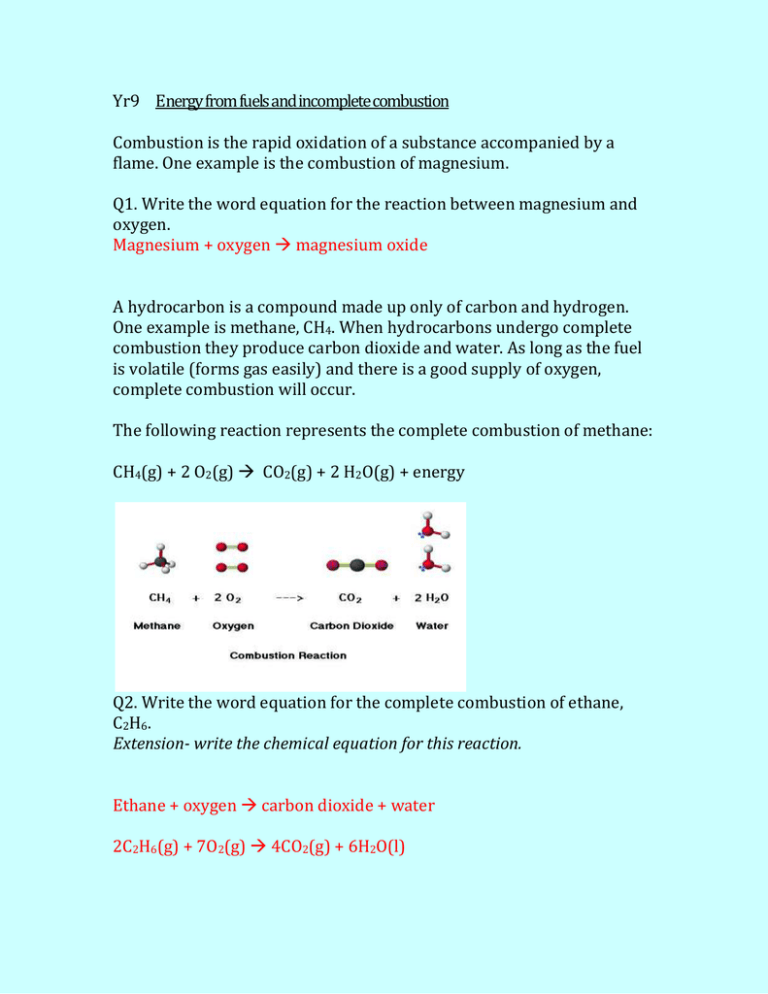
The combustion of methane CH4 produces carbon dioxide CO2 and water.
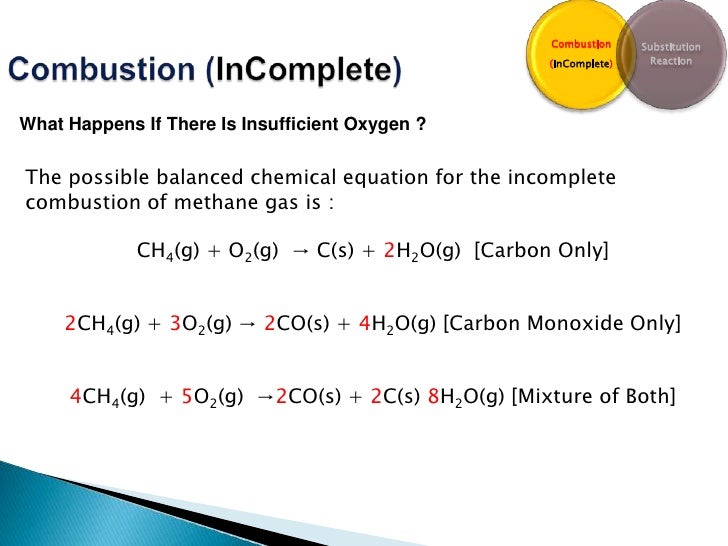
Incomplete combustion of ch4. Incomplete combustion means burning in a lack of air. CH4g 2O2g CO2g 2H2Og A common misconception is that carbon monoxide is the only product of the incomplete combustion of a hydrocarbon. This gives us carbon dioxide and Hydrogen as the final products.
Complete Combustion vs Incomplete Combustion. Ususally the incomplete combustion is formed this way. It is observed that Methane employs 2 molecules of oxygen.
The balanced equation for the incomplete combustion of methane is. Additionally what are the products for the combustion of methane ch4. Methane CH4 burns completely in oxygen to yield carbon dioxide and water.
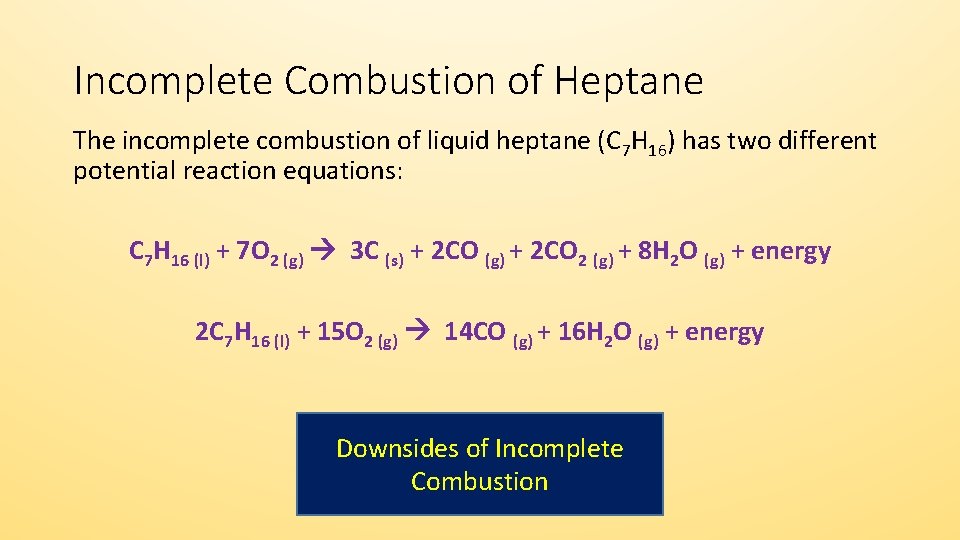
Incomplete Combustion Incomplete combustion occurs when there is not enough oxygen for the fuel to fully react. 2 CH4 g 3 O2 g 2 CO g 4 H2O g Like all hydrocarbons the combustion of methane forms water due to the presence of hydrogen but produces carbon dioxide as a result of complete combustion and carbon monoxide dangerous as a result of incomplete combustion. Determine the excess.
The combustion of methane is represented by the equation. The complete combustion of a hydrocarbon produces carbon dioxide gas and water vapor. Instead the reaction will leave behind carbon monoxide and soot.
Available for all the carbon to turn into carbon dioxide. If there is not enough oxygen. CH4g 2 O2g CO2g 2 H2Og Methane here is the reducing agent that reacts with oxygen which is the oxidizing agent.





:max_bytes(150000):strip_icc():format(webp)/methanecombustion-58e3e6005f9b58ef7e0daa10.jpg)