Nice Sodium Polyacrylate And Water Chemical Equation
It has the capacity to absorb and.
Sodium polyacrylate and water chemical equation. With a superior absorbencysodium polyacrylate SAP can absorb several hundred-times its own weight in water. It starts out as a powder and as it comes into contact with water it. The answer would be clear when we know the mechanism why sodium polyacrylate absorbs water.
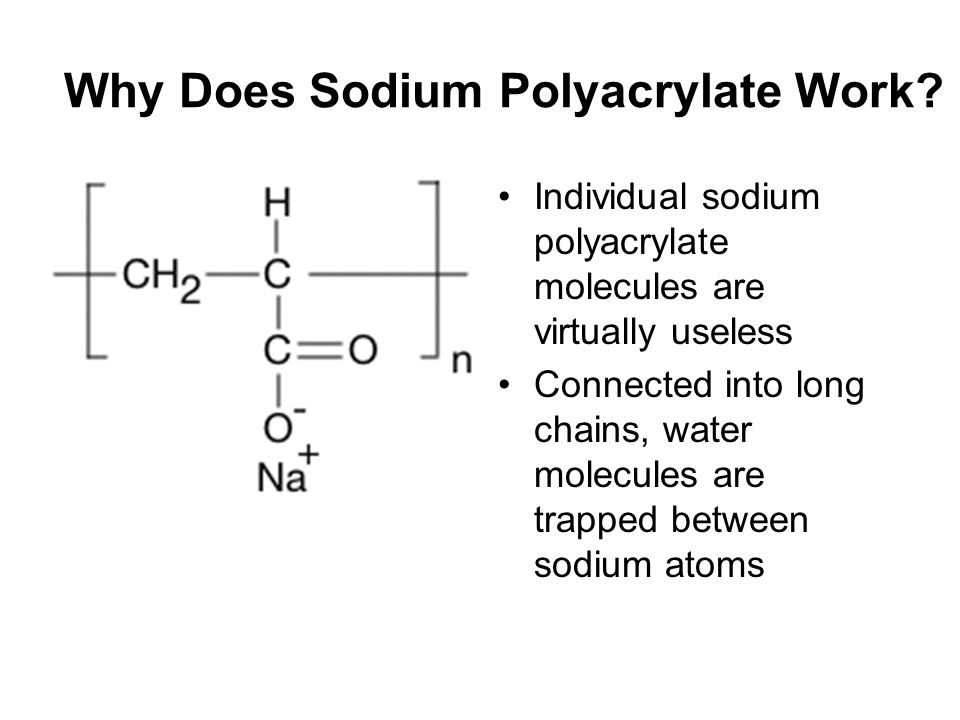
The sodium ion dissociates in water leaving 2 charged particlesa free sodium cation and a carboxylate CO 2 ionattached to the strand. Sodium polyacrylate is an example of a super-absorbing polymer. Chemical equation for sodium polyacrylate and water tessshlo osmosis magic sed 695b you polyaspartate wikipedia the use of super absorbent polymer as blocker in concrete structures absorbing materials absorb about 300 800 times its weight.
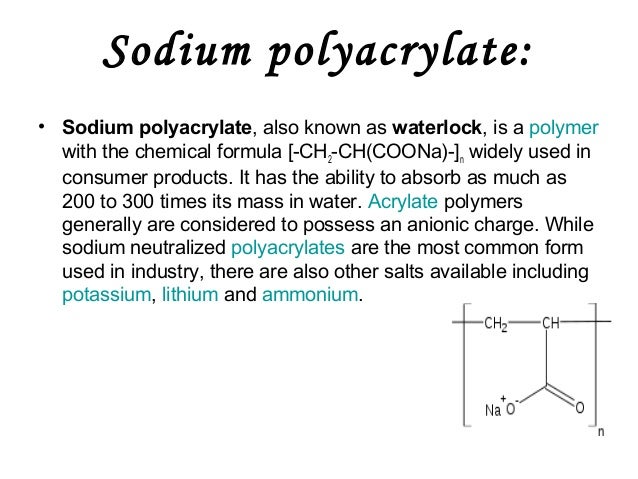
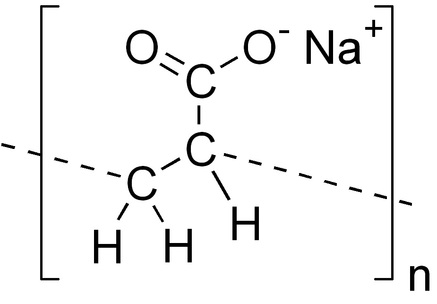
The Sodium Polyacrylate polymer shown below in blue contains a polar charge that allows it to combine with other liquids by forming hydrogen bonds. As a non-toxicharmless nonpolluting polymer absorption material sodium polyacrylate is widely used in a variety of common products from diapers to fake snow. Sodium polyacrylate also referred to as Slush Polymer with the chemical formula CH 2 CHCO 2 Na n.
Discussion Conclusion Appears to be physical however is chemical Our research suggests that sodium polyacrylate can absorb up to 300 time its weight in tap water It is mainly due to its polymers SAPs super absorbent polymers. This page is about the good water-absorbing material Sodium polyacrylate uses. Chemical Equation For Sodium Polyacrylate And Water Tessshlo.
FIRST-AID MEASURES Eyes Rinse with plenty of water for at least 15 minutes and seek medical attention. What is the effect of sodium polyacrylate on the temperature of the chemical mixed with water. Chemical equation for sodium polyacrylate and water tessshlo osmosis magic sed 695b you polyaspartate wikipedia super absorbent material absorbing materials absorb about 300 800 times its weight polymer swelling performance of poly acrylamide co acrylic acid potassium salt.
Why is so absorbent. It has the ability to absorb as much as 200 to 300 times its mass in water. The product of this reaction is a long-chain copolymer consisting of alternate units of acrylic acid and sodium acrylate.