Beautiful Work Word Equation For Rusting Of Iron Class 7

The general equation for this reaction is.
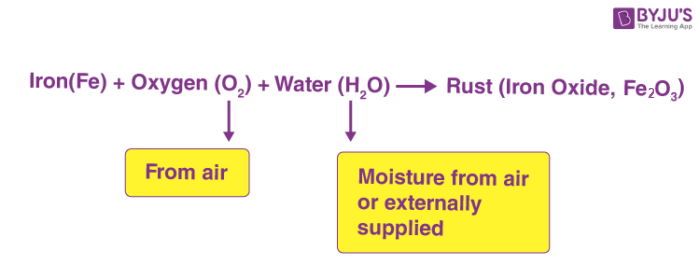
Word equation for rusting of iron class 7. The iron reacts with water and oxygen to form hydrated iron III oxide which we see as rust. Class 7 Chemistry Physical and Chemical Changes. If polished iron nails are kept in three separate test tubes state the contents in each test tube required to prove conditions for rusting.
Chemical Changes Class 7 Physical And Chemical Changes Science. Iron water oxygen. Rusting of Iron Physical and Chemical Changes - Get topics notes Online test Video lectures Doubts and Solutions for CBSE Class 7 on TopperLearning.
Conditions Necessary for Rusting. If polished iron nails are kept in three separate test tubes state the contents in each test tube required to prove conditions for rusting. Rusting of iron refers to the formation of rust a mixture of iron oxides on the surface of iron objects or structures.
Jul 282021 - Rusting of iron s equation. Rust is a hydrated form of a compound known as ironIIIoxide. What Causes Rusting And Is It Limited To Iron Glamblog.
Two conditions are necessary for the rusting of iron to take place. Rust is iron oxide Fe 2 O 3. Here is the word equation for the reaction.
Rust is hydrated ferric oxide Fe 2 O 3xH 2 O which forms a reddish brown coating over iron. Rust is a general term that defines a series of iron oxides red oxides. This phenomenon is called rusting of iron.



:max_bytes(150000):strip_icc()/BalanceEquations1-56a132765f9b58b7d0bcf535.png)