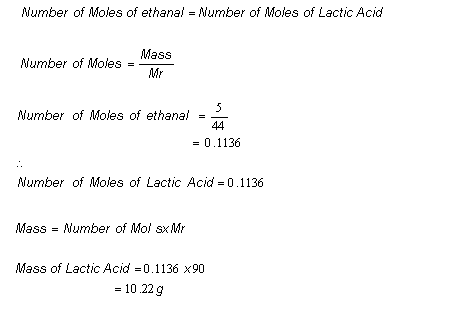Favorite Yield Of Reaction Calculator

We use the molar ratio of reactant in a balanced chemical reaction to understand how much product will be created under ideal conditions.
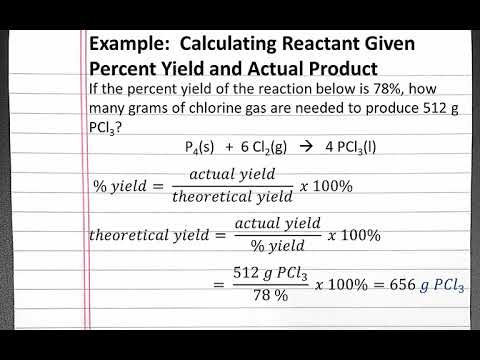
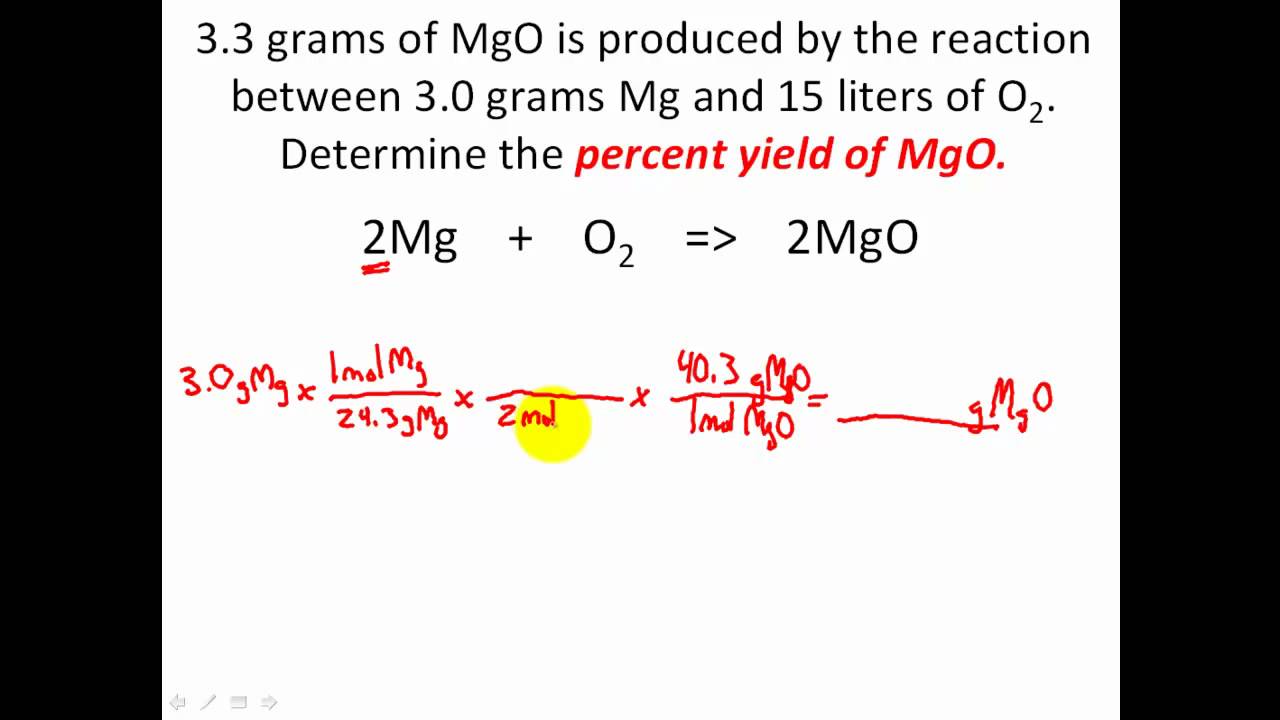
Yield of reaction calculator. A percent yield also referred to as a percentage yield is the ratio of the actual yield of mass of a chemical reaction to the theoretical yield that the reaction should produce. In very rare cases chemical reactions occur completely where no reactants get wasted. In chemistry terms we refer to yield as the quantity of product that can be produced from a reactionTheoretical yield is the maximum amount of product that could.
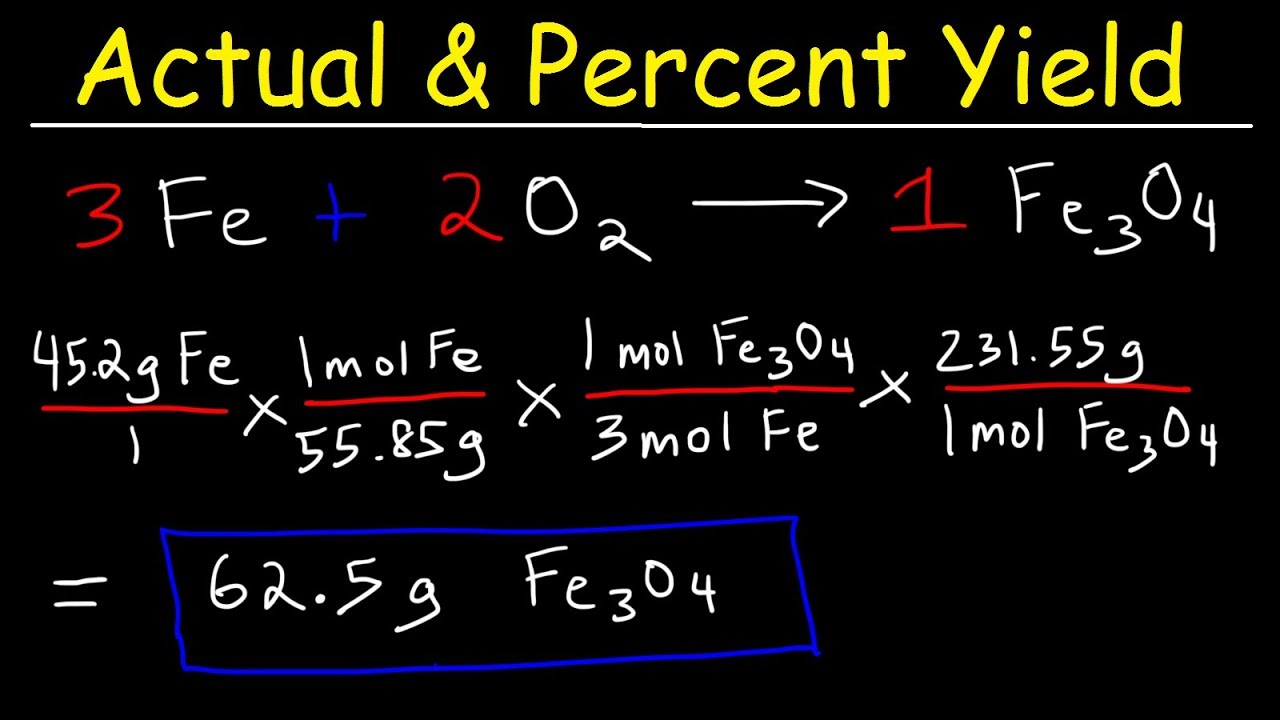
By following them we can calculate how many grams of product each reagent can produce. There are a few steps. The number of products formed in this kind of reaction is a theoretical yield.
Theoretical yield is the amount of product obtained mathematically. Is the maximum possible mass. Determine the theoretical yield of the reaction Yt.
Isotope Patterns from your choice of formula. Convert the result obtained in step 2 to the same units as the theoretical yield. Since the theoretical yield is a measurement of the highest possible amount of the desired product that is expected from a reaction the limiting reactant has to be considered when calculating it.
It can be calculated from. Determine the mole ratio between the reactants and the products. To calculate a reactions percent yield follow these steps.
Chemical equations must be balanced equations. With these two pieces of information you can calculate the percent yield using the percent-yield formula. Otherwise you will need to identify the other byproducts and adapt your reaction.