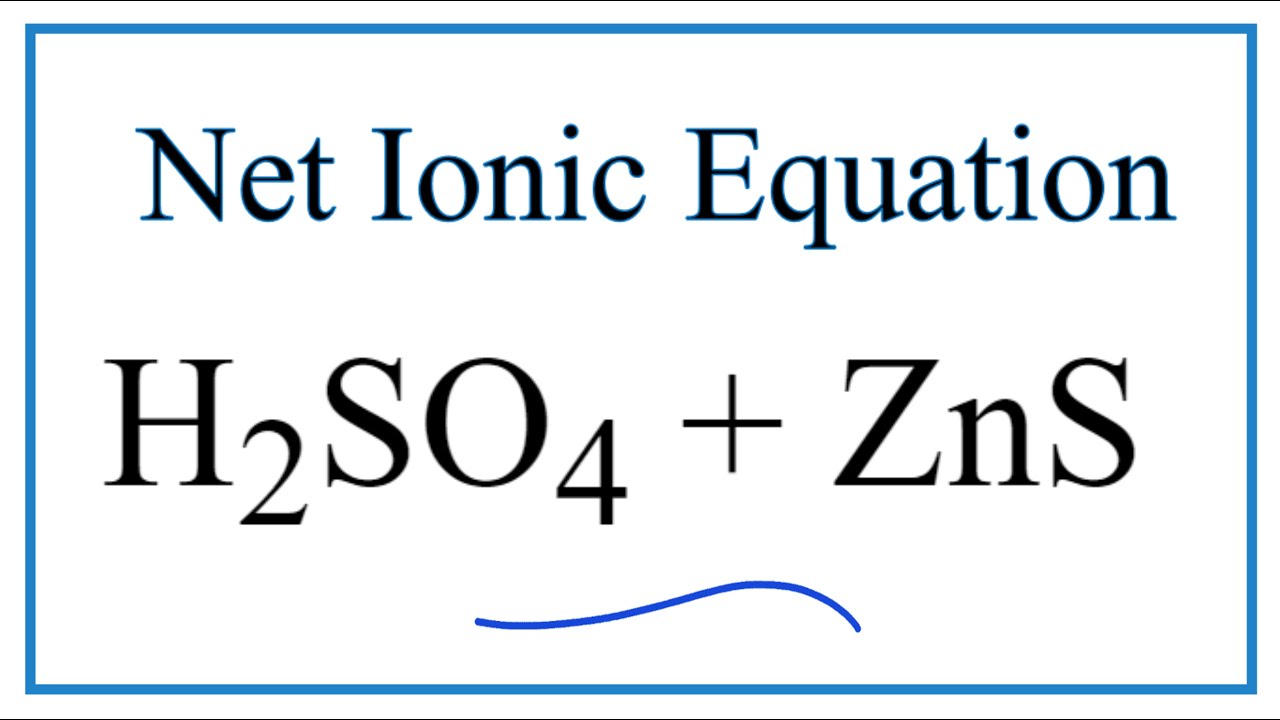Smart Zinc And Sulfuric Acid Net Ionic Equation

Zinc and sulfuric acid net ionic equation.
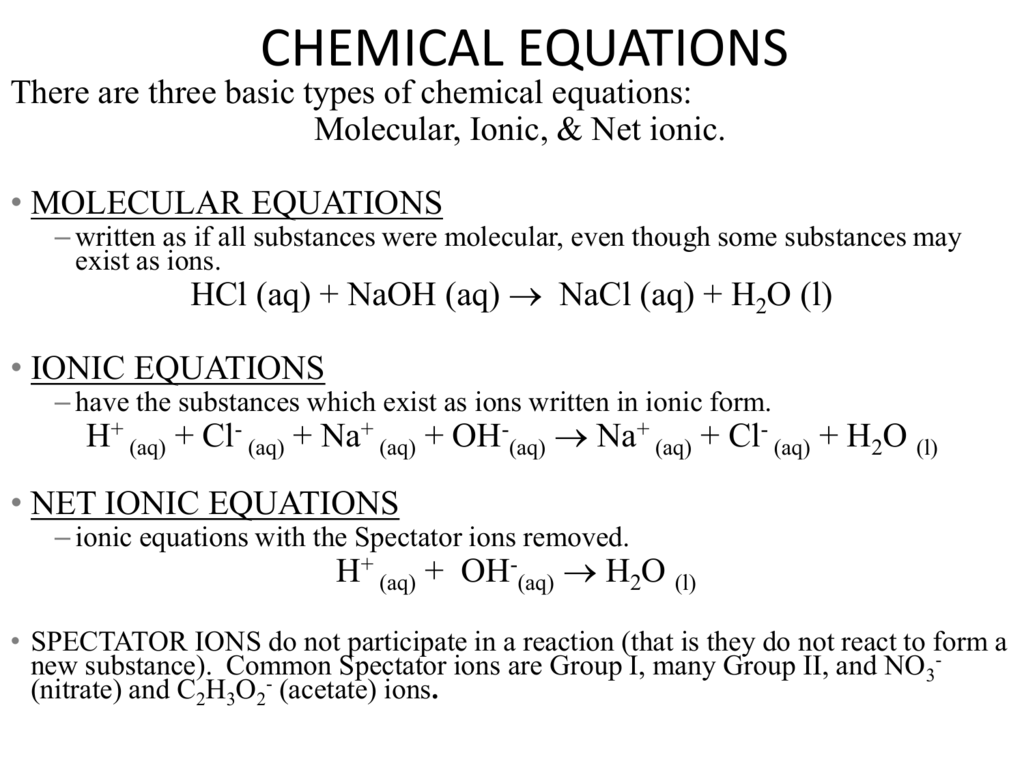
Zinc and sulfuric acid net ionic equation. Since there are no spectator ions nothing is eliminated and the net ionic equation is the same as the complete ionic equation. H2SO4 aq 2H aq SO42- aq Part D Net ionic equation. Name the products of the reaction between zinc and sulfuric acid and write an equation for the reaction.
Zinc ion and hydrogen gas form when zinc metal reacts with. CaCO3s H2SO4aq CaSO4s H2Ol CO2g The arrow written next to CO2 indicates that this product escapes as a gas. The Balanced Equation For Zinc Nitrate And Sulfuric Acid is Zn NO32H2SO4ZnSO42HNO3.
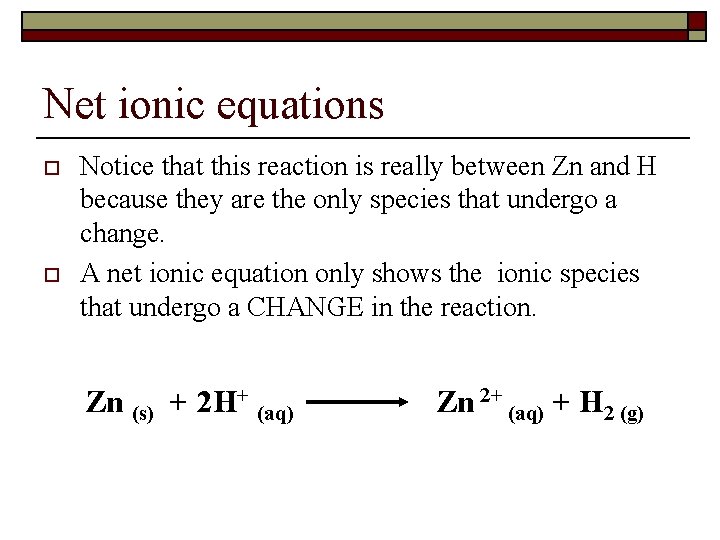
Write the total ionic and net ionic equation for the following reactions. The reaction would be MUCH more rapid with hydrochloric acid which follows the same stoichiometry What happens when zinc metal was mixed with hydrochloric acid. When zinc reacts with hydrochloric acid it produces zinc.
The net ionic equation is CaCO3 H2SO4 CaSO4 CO2 H2O. The word equation is. Something is initially missing sulphate ion which will be with the zinc ion as zinc sulphate.
The redox reaction between zinc metal and sulfuric acid produces zinc sulfate and hydrogen gas. Dilute sulfuric acid is added to solid calcium fluoride. For the neutralization reaction between any monoprotic strong acid and strong base the resulting net ionic equation will be the same as that shown above for HCl and.
Carbonic acid which is produced during this reaction in unstable. H SO42- CaF2 Æ CaSO4 HF 34. The products are zinc chloride and hydrogen.