Favorite Chemical Equation For Propane Combustion

Answer to The combustion of propane may be described by the.
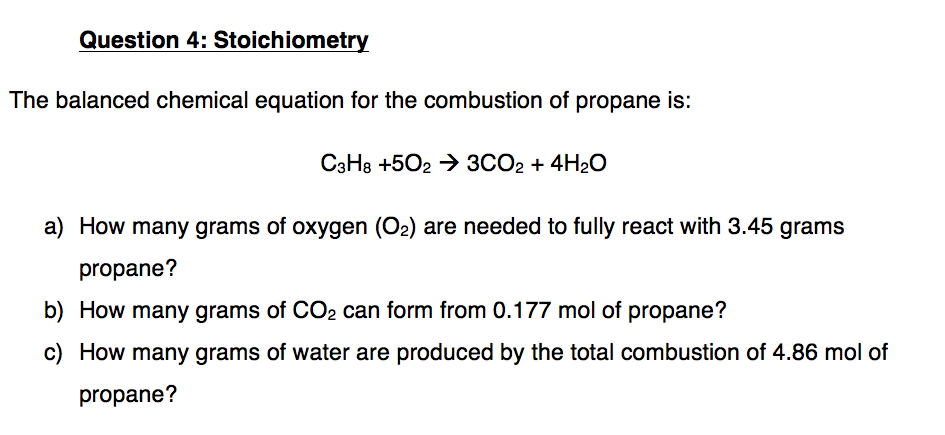
Chemical equation for propane combustion. Combustion is the reaction with oxygen to form carbon dioxide CO2 and water H 2O the opposite of photosynthesis. Propane is a gas at room temperature and pressure but becomes a liquid when compressed. The combustion of propane may be described by the chemical equation CH 50 300 4H09 How many grams of O g are needed to completely burn 21 g C H g.
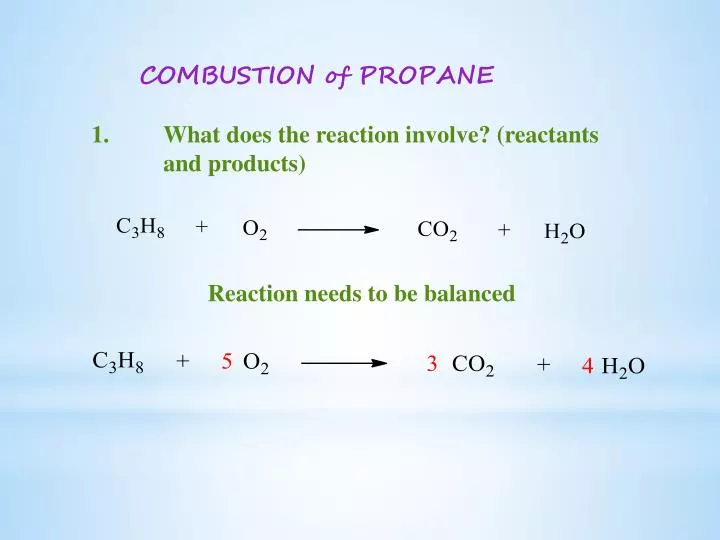
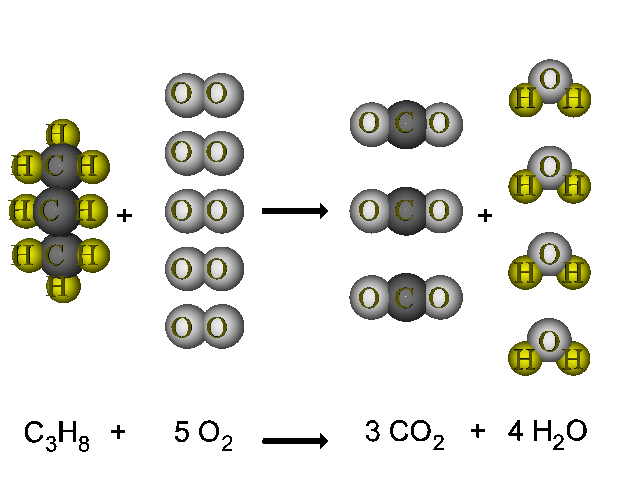
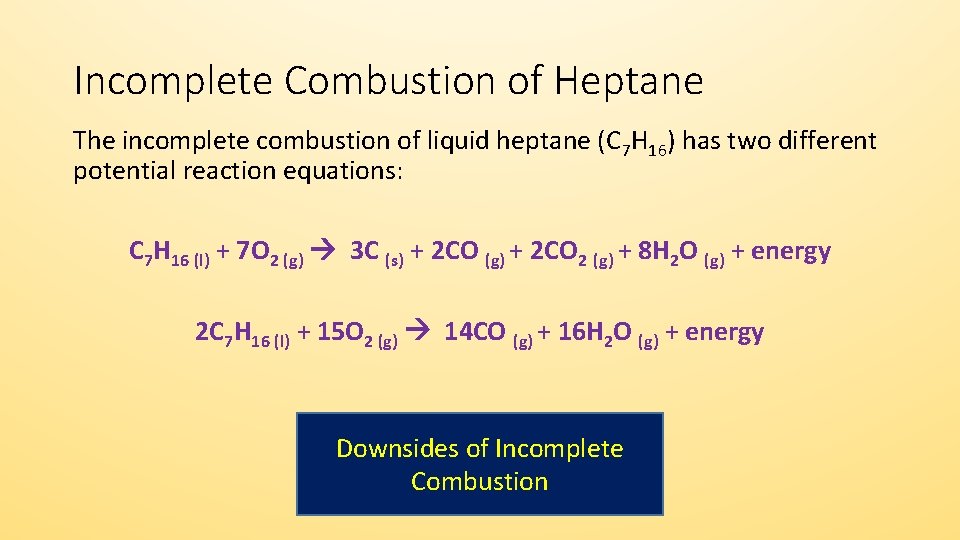
This is a perfect example of a combustion reaction because we have a carbon based compound reaction with oxygen gas to produce carbon dioxide and water. When not enough oxygen is present for complete combustion propane burns to form water and carbon monoxide. C3H8 g 5O2 g 3CO2 g 4H2O g energy propane oxygen carbon dioxide water energy Watch Video 1 at the bottom of the page for a brief visual explanation of this process.
If we write that as a chemical equation with the chemical formula for each substance itll be one C-3-H-8 plus five O-2 reaction arrow Three C-O-2 plus four H-2-O. C 3 H 8 3. So our equation is C3H 8.
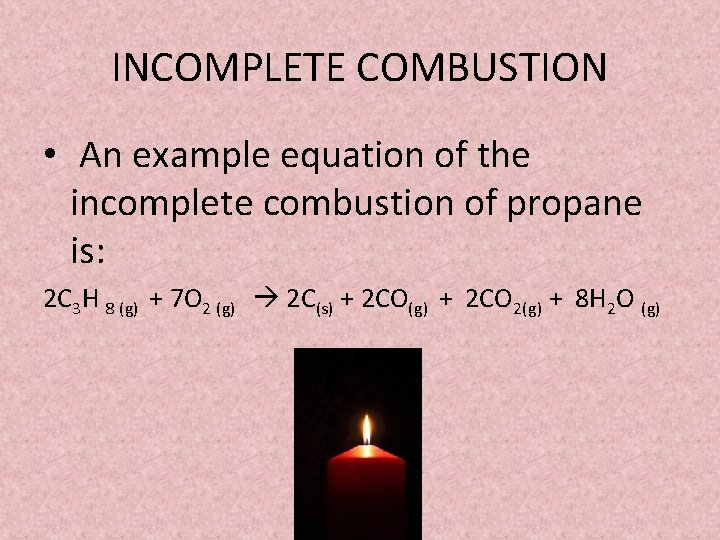
The incomplete combustion of propane C 3 H 8 produces two possible balanced equations. The chemical equation involved in the combustion of propane is as follows. Eq C_3 H_8left g right O_2left g right to C O_2left g right.
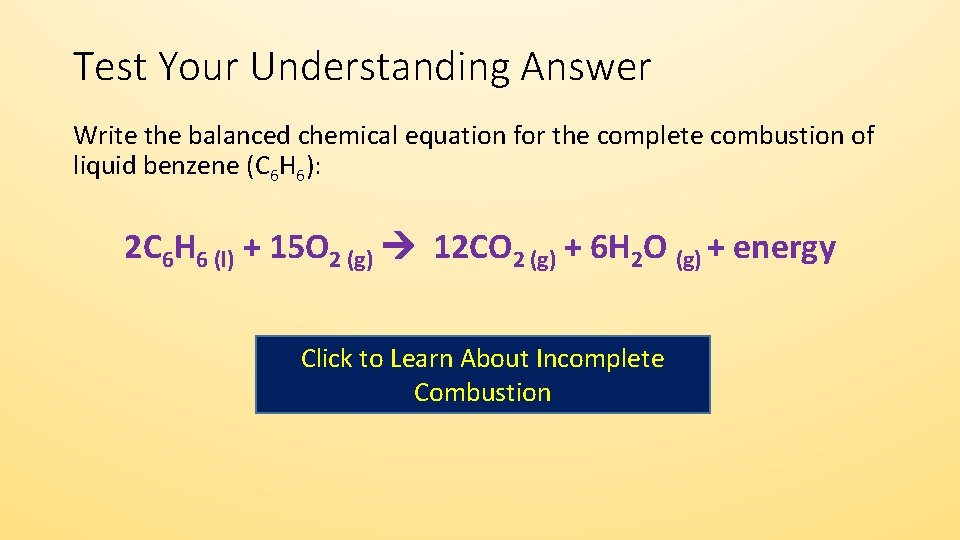
Find step-by-step Biology solutions and your answer to the following textbook question. Incomplete combustion of propane yields carbon carbon dioxide and water. The result of incomplete combustion is once again water vapour carbon dioxide and heat.
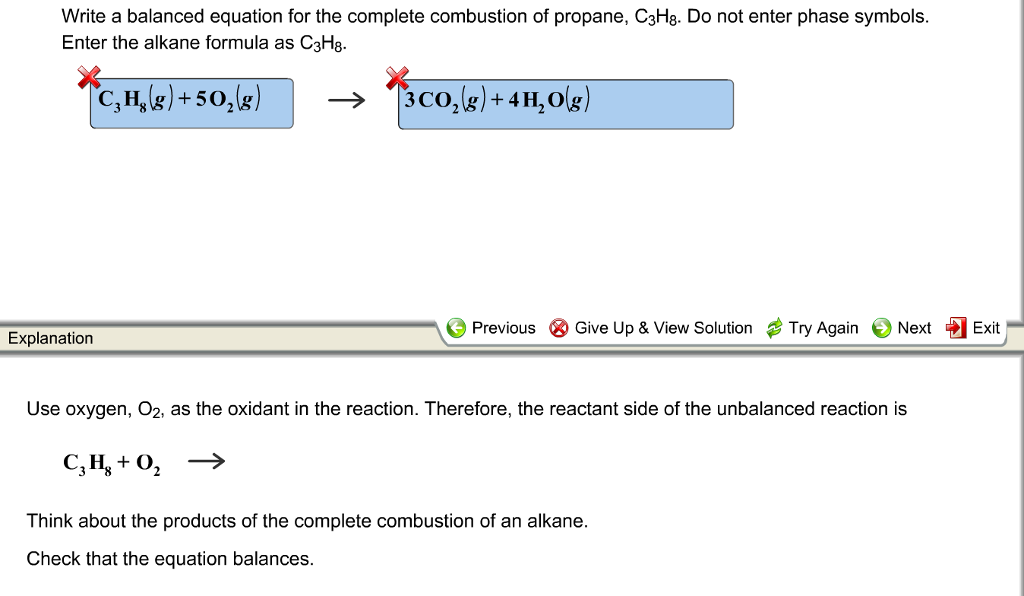
A Write the general equation for the complete combustion of a hydrocarbon. In the presence of excess oxygen propane burns to form water and carbon dioxide. The required equation for the combustion of propane is given as.