Casual Incomplete Combustion Of Acetylene Equation
Incomplete combustion of acetylene gas.
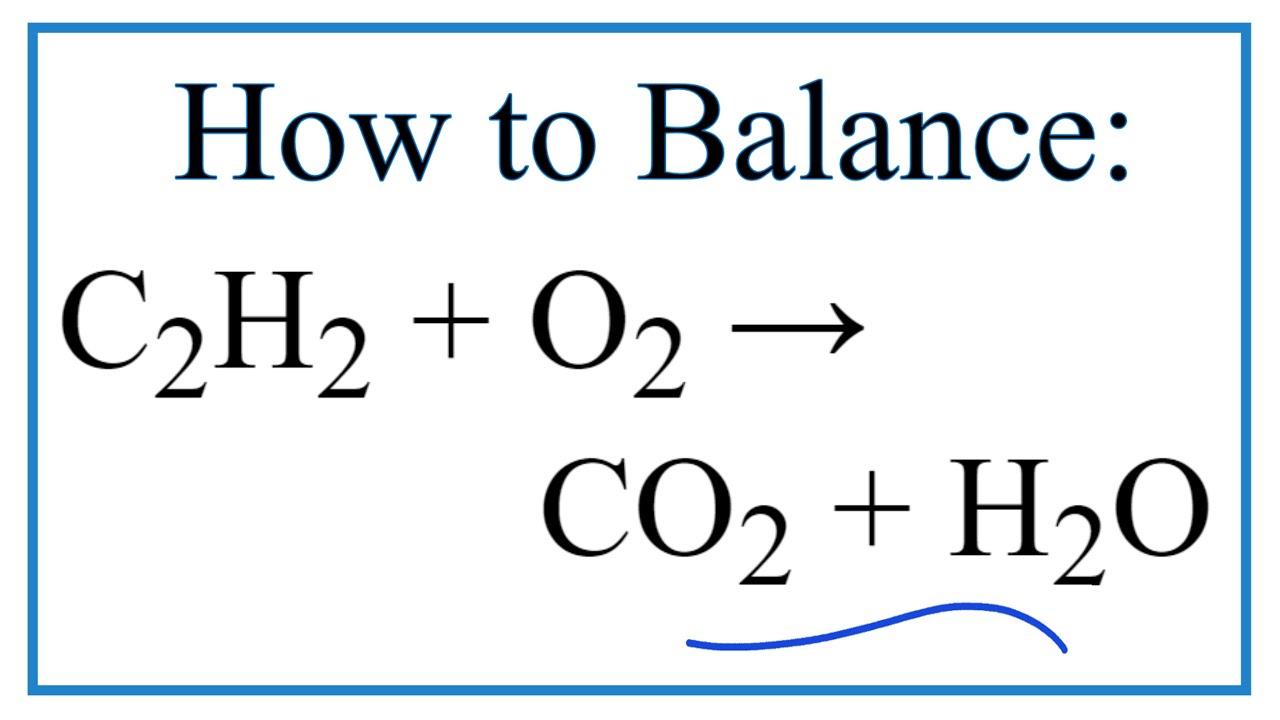
Incomplete combustion of acetylene equation. Calcium carbide reacts with water to form acetylene. A combustion reaction occurs when a compound merges with oxygen to release heat in an exothermic reaction. 2C 2 H 2 3O 2.
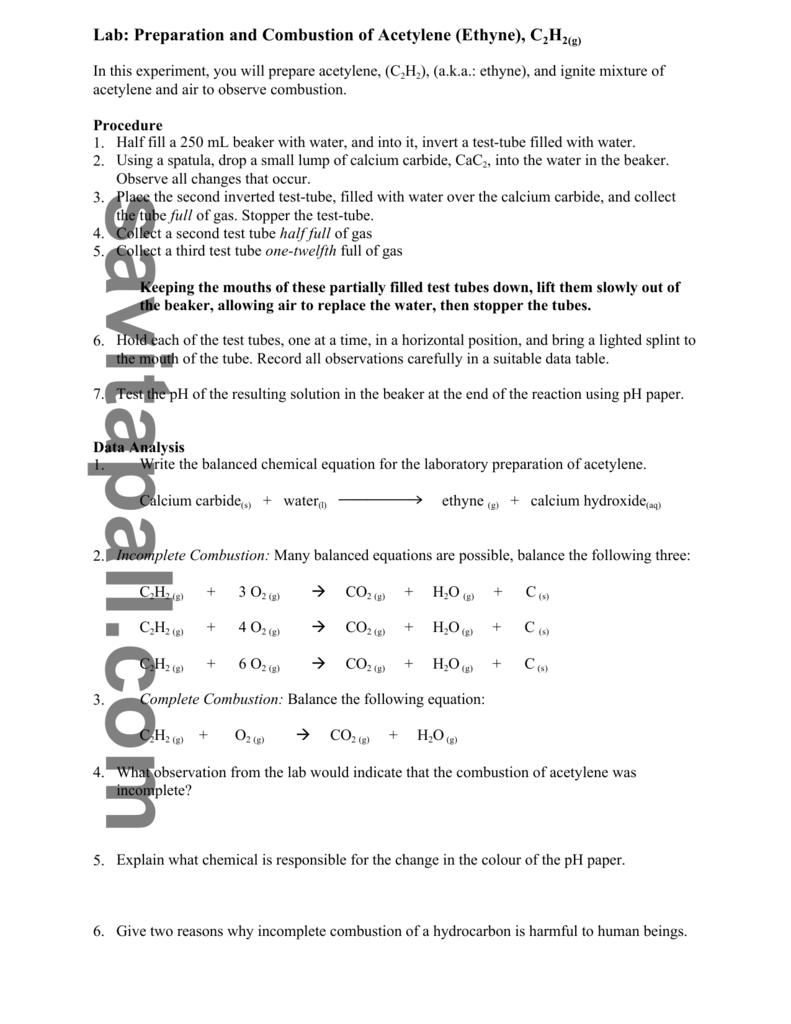
When this happens incomplete combustion results in the dangerous build-up of poisonous carbon monoxide gas shown in the following equation for acetylene. The O is from the. Improving primary secondary and tertiary air distribution this includes verification when a boiler is commissioned or after major modifications.
Incomplete combustion is generally due to poor mixing of the air and fuel insufficient residence time insufficient temperature and low total excess air. The equation is specific to the fuel but the general form is. 4 CO2 2 H2O.
Phase symbols and energy changes are optional. If you have a higher ratio than that combustion will be incomplete. Because so many reaction products are possible incomplete combustion cannot be represented by a single chemical equation.
What is the balanced. Based upon this answer explain why there is a high hazard for carbon monoxide CO poisoning from gas stoves and vehicles that are not well-maintained. 6CH2 150212C02 6H2O.
If not enough oxygen is present for complete combustion incomplete combustion occurs. The balanced equation for the combustion of acetylene is 2 H2C2 5 O2. For incomplete combustion there are two possibilities.